Acids and Bases identification
advertisement

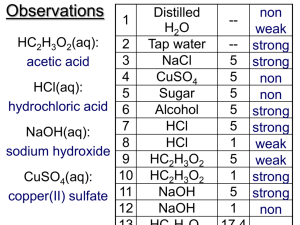
Mr. O Lab 25 Acids and bases Name: Materials: 6M HCl, 6M HC2H3O2, .5M NaOH, Mg, Fe, Al, Cu, and Zn Procedure: Part A Data Table Red Blue PH Bromothymol Phenolphthalein litmus Litmus Paper Blue 6M HCl 6M HC2H3O2 .5M NaOH Part B Use the following well tray place the metals in separate wells. Test each metal with the two acids in the table. Comparative reactivity (very fast, fast, slow, very slow) 6M HCl 6M HC2H3O2 Zn Mg Fe Al Cu Equations: Write the following equations 1. Each metal with HCl 2. Each metal with HC2H3O2 Conclusions and questions 1. What type of reaction occurs between a metal and an acid? Write a general equation for this type of reaction? 2. Explain the difference in reaction rates between the two different acids. 3. Why does Cu look as if it reacted? 4. What type of gas is liberated during a acid metal reaction? 5. What type of acid is normally found in rain? Acid rain?