Instructor`s Copy
advertisement

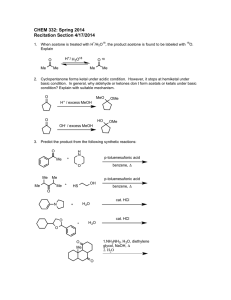
Instructor’s Copy Lab Worksheet – Speed It Up Table 1: Effect of Concentration on Rate of Reaction Concentration of HCl Time for complete reaction (seconds) 3M HCl Should be faster. 1M HCl Should be slower. Table 2: Effect of Temperature on Rate of Reaction Temperature of HCl Time for complete reaction (seconds) Hot Should be fastest. Cold Should be slowest. Room-temperature Should be medium. Data Questions: 1. One trial is not really going to give us accurate data. What could we have done in this lab to improve the accuracy of our data? Do more trials. 2. What effect does concentration of the acid have on the rate of the reaction of HCl and Mg? The higher the concentration, the faster the reaction. 3. What is the effect of the temperature of the acid on the reaction rate of HCl and Mg? The higher the temperature, the faster the reaction. 4. Write the balanced equation for the reaction between HCl and Mg. 2 HCl + Mg MgCl2 + H2 5. What would increasing the surface area of magnesium, for example using many small pieces instead of 1 large piece, do to the reaction rate? It would react faster because more of the magnesium is in contact with the acid. Conclusion: The purpose of this experiment was to observe the effect of concentration and temperature on the rate of reaction. Two other factors that effect the rate of a reaction are surface area and catalysts. Increasing the surface area will increase the contact between the reactants and will increase the rate of the reaction. Increasing the concentration increases the rate of reaction. A catalyst is a substance that changes the rate of the reaction without being changed itself. In general, when we increase the contact between the particles, we increase the rate of the reaction. Conversely, if we need to slow down the rate of a reaction, we should decrease the contact between the particles.