CHEM 332: Spring 2014 Recitation Section 4/17/2014 H /
advertisement

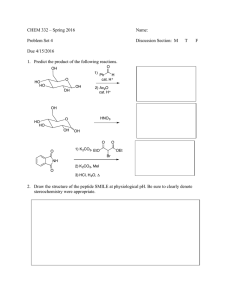
CHEM 332: Spring 2014 Recitation Section 4/17/2014 + 18 1. When acetone is treated with H /H2O , the product acetone is found to be labeled with Explain H + / H 2O18 O Me Me 18 O. O 18 Me Me 2. Cyclopentanone forms ketal under acidic condition. However, it stops at hemiketal under basic condition. In general, why aldehyde or ketones don t form acetals or ketals under basic condition? Explain with suitable mechanism. O MeO H + / excess MeOH O HO OH- / excess MeOH OMe OMe 3. Predict the product from the following synthetic reactions: O H N p-toluenesufonic acid Me benzene, Δ O Me Me Me p-toluenesufonic acid Me OH HS benzene, Δ O cat. HCl H 2O N O H 2O cat. HCl O 1.NH 2NH 2, H 2O, diethylene glycol, NaOH, Δ 2. Η2Ο O Me O 4. Dihydropyran reacts readily with an alcohol in the presence of a trace of anhydrous HCl to form a tetrahydropyranal (THP) ether. ROH HCl O Dihydropyran O OR Tetrahydropyranyl ether (a) Write a plausible mechanism for this reaction. (b) THP ethers are stable in aqueous base but hydrolyze rapidly in aqueous acid to yield original alcohol and another compound Explain. (What is the other compound?). (c) The Tetrahydropyrany group can be used as a protecting group for alcohols. Show how you might use it in a synthesis of 5-methyl-1, 5-hexanediol starting with 4-chloro-1-butanol. 5. Predict the product and draw a arrow-pushing mechanism for it. cat. HCl N H 2O