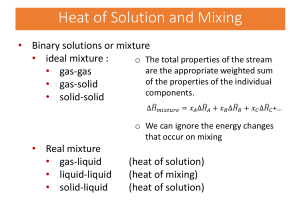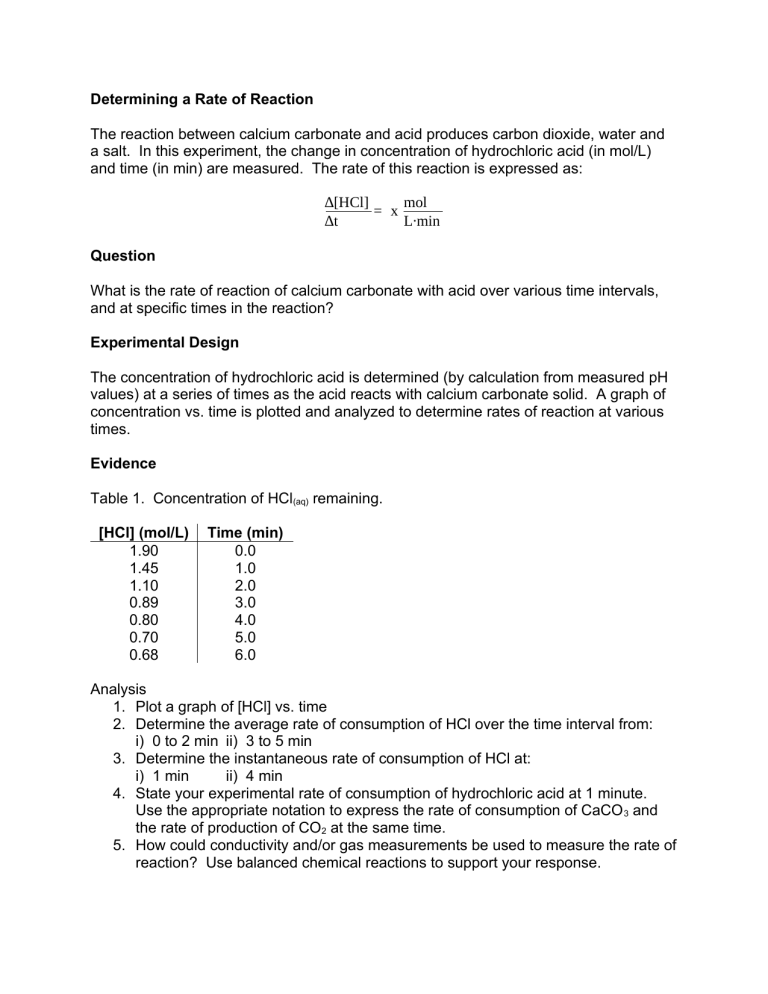
Determining a Rate of Reaction The reaction between calcium carbonate and acid produces carbon dioxide, water and a salt. In this experiment, the change in concentration of hydrochloric acid (in mol/L) and time (in min) are measured. The rate of this reaction is expressed as: ∆[HCl] mol = x ∆t L∙min Question What is the rate of reaction of calcium carbonate with acid over various time intervals, and at specific times in the reaction? Experimental Design The concentration of hydrochloric acid is determined (by calculation from measured pH values) at a series of times as the acid reacts with calcium carbonate solid. A graph of concentration vs. time is plotted and analyzed to determine rates of reaction at various times. Evidence Table 1. Concentration of HCl(aq) remaining. [HCl] (mol/L) 1.90 1.45 1.10 0.89 0.80 0.70 0.68 Time (min) 0.0 1.0 2.0 3.0 4.0 5.0 6.0 Analysis 1. Plot a graph of [HCl] vs. time 2. Determine the average rate of consumption of HCl over the time interval from: i) 0 to 2 min ii) 3 to 5 min 3. Determine the instantaneous rate of consumption of HCl at: i) 1 min ii) 4 min 4. State your experimental rate of consumption of hydrochloric acid at 1 minute. Use the appropriate notation to express the rate of consumption of CaCO 3 and the rate of production of CO2 at the same time. 5. How could conductivity and/or gas measurements be used to measure the rate of reaction? Use balanced chemical reactions to support your response.