group 1 = 1+ group 15 = 3-
advertisement

Naming Ionic Compounds:
An ionic bond is the bonding of _______, held together by the ___________________ force.
Example:
Na (sodium) gives one electron to Cl (chlorine) resulting in the compound NaCl (sodium chloride)
Pattern on Periodic Table for (MOST COMMON) Oxidation Numbers (charges):
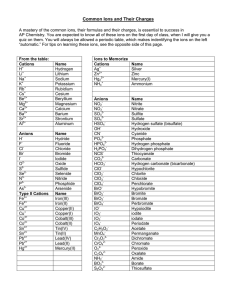
group 1 = 1+ (1 ve)
group 2 = 2+ (2 ve)
groups 3-12 = see below
group 13 = 3+
(3 ve)
group 15 = 3group 16 = 2group 17 = 1-
(5 ve)
(6 ve)
(7 ve)
group 18 = 0 (8 ve)
group 14 = see below
Cation:
always named first
usually a metal (exceptions: hydrogen ion = H+ / ammonium ion = NH4+)
Need to include “ion” after the name to indicate the ion, not the atom
example:
Na = sodium (atom)
Na+ = sodium ion
use pattern on periodic table (above) for oxidation numbers
see table for transition elements and group 14 - memorize ions that are bolded:
groups 3-12;
Ag = always 1+ (no roman numeral)
Zn = always 2+ (no roman numeral)
Cu = 1+ or 2+ {copper (I) or copper (II)}
Fe = 2+ or 3+ {iron (II) or iron (III)}
group 14:
Sn = 2+ or 4+ {tin (II) or tin (IV)}
Pb = 2+ or 4+ {lead (II) or lead (IV)}
If and only if the cation may have more than one charge, the name will include a roman numeral
after the name of the cation (see above)
Anion:
always named second
non-metal or polyatomic:
o nonmetal: change ending to “ide”
o use pattern on periodic table (above) for oxidation numbers
o
polyatomic: usually end in “ate” or “ite” (exceptions: CN- = cyanide / OH- =
hydroxide)
see table for charges – memorize ions that are bolded:
o
** go over hints in naming…
o
Write the formulas and oxidation numbers for the following polyatomic ions:
ammonium:
hydroxide:
cyanide:
acetate:
chlorate:
nitrate:
sulfate:
carbonate:
phosphate:
From the “ate” form of the ion above, write the formulas and ox. #s of the following ions:
perchlorate:
chlorite:
hypochlorite:
nitrite:
sulfite:
hydrogen sulfate:
hydrogen carbonate:
bicarbonate:
hydrogen phosphate:
dihydrogen phosphate: