Matter Matters! Hydrogen Oxygen 1 proton
advertisement
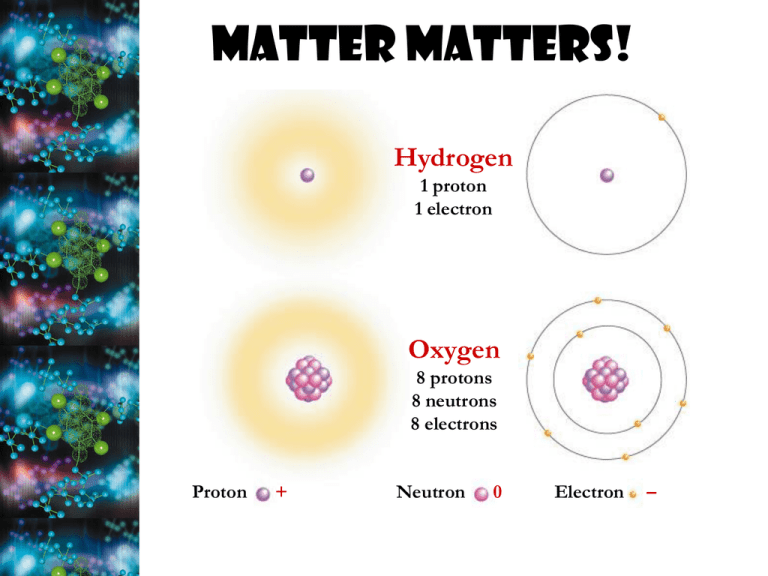
Matter Matters! Hydrogen 1 proton 1 electron Oxygen 8 protons 8 neutrons 8 electrons Proton + Neutron 0 Electron – The Periodic Table K C Na Mg Ca N O P S Interactions between atoms Electrons are key! • chemical behavior of an atom depends on # valence electrons What’s the magic number?? A little more on electrons… What do you notice about the # of electrons in each element? Elements in same row have same # shells & those in same column have same valence & chemical properties Bonds to know… Little heat needed to break hydrogen bonds attraction between + and – hydrophobic & hydrophilic interactions interaction with H2O ionic bonds (transfer of electrons) Van der Waals forces Inter or intramolecular attractions (forces of millions of H2O molecules, Ar2, (HF2)) Sharing of electrons covalent bonds(nonpolar, polar) Van der Waal(sum of the attraction and repulsion forces between molecules) Collectively, such interactions can be strong, as between molecules of a gecko’s toe hairs and a wall surface • Chemical reactions are the making and breaking of chemical bonds • The starting molecules of a chemical reaction are called reactants • The final molecules of a chemical reaction are called products • Chemical equilibrium is reached when the forward and reverse reaction rates are equal Energy! & Reactions What is Energy? • capacity to do work/supply heat •Many faces of energy: • Potential energy (EP) – stored • Kinetic energy (EK) – motion; aka thermal energy of molecular motion • amount of thermal energy in an object is its temperature EP high EP transformed to EK Original EP transformed: 1. Mechanical energy breaks rocks 2. Heat; thermal energy increases rock Same type of energy temp transfer occurs within atoms and 3. Sound drives reactions LawS of Thermodynamics • First - Energy NEVER created or destroyed – changes form • Second – entropy always increases in an isolated system **Entropy – amount of order, disorder, of chaos in a system (∆S) Ex: “a neat room is more organized but less stable than a messy room, which is disorganized but more stable” (Mader page 106) Spontaneity of Reactions • Spontaneous! – ONLY when products have ↓ EP than reactants AND products have ↑ entropy Spontaneous nail rusting, wood burning Nonspontaneous: recharging a battery • Spontaneous reactions are exergonic (exothermic); nonspontaneous rxns are endergonic (endothermic) – require LARGE amt of energy Redox reactions • Paired rxns that involve (-) or (+) of electrons **Follow the H’s in living systems!** Oxidation Atom loses Electron Remove H’s Reduction Atom gains Electron Add H’s • LEO the lion goes GER! ol x al e se i ie d ecd ncu t i t c r z r e o e o n n Example: Cellular Respiration Oxidation Reduction Glucose is oxidized (loss of electrons) and oxygen is reduced (gains electrons) C6H12O6 is high energy molecule and CO2 and H2O are low energy which is why energy is released Versatility of Carbon! • 4 covalent bonds (to itself and other atoms) • single, double, triple bonds •Form straight and branched chains along with rings •Nearly endless variety & complexity •Can form polymers (protein, carbs, nucleic acids) • Structural isomers Drug Condition Ibuprofen Pain; inflammation Albuterol Effective Enantiomer Ineffective Enantiomer S-Ibuprofen R-Ibuprofen R-Albuterol S-Albuterol Asthma STRUCTURE DETERMINES FUNCTION! Functional Groups-they direct the chemical behavior of a compound Has acidic properties Acts as a base Is polar and can form hydrogen bonds with H2O Found in sugars At end of carbon skeleton Within carbon skeleton Contributes a negative charge, can react with H2O releasing energy 2 groups can form cross-linking Methyl (CH3) – can affect the expression of genes (attached to DNA) Water, water, everywhere • H2O molecules form H-bonds with one another – +H attracted to –O creates a sticky molecule Elixir of Life • Polarity allows for… 1. cohesion & adhesion • surface tension, capillary action 2.good solvent • many molecules dissolve in H2O • hydrophilic vs. hydrophobic 3.lower density as a solid • ice floats! 4.high specific heat • water stores heat 5. high heat of vaporization • heats & cools slowly What’s the Water Property? Direction of water movement Figure 3.3 Adhesion Two types of water-conducting cells Cohesion Direction of water movement 300 m







