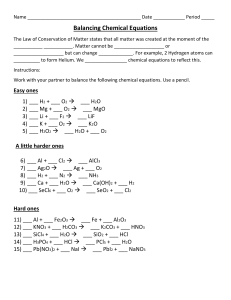
B a l a n c i n g ...
advertisement

B a la n c in g R e d o x E q u a ito n s 1. Write separate equations for reduction and oxidation half reactions 2. For Each half reactions a. First Balance all elements except O and H b. Add H2O to balance O c. Add H+ to balance H d. add electrons to balance net charge 3. If necessary multiply one or both ½ reaction equations by integer so the total number of electrons used in one reaction is equal to the total number of electrons furnished by the other. 4. Add the two ½ reaction equations together and cancel any common terms 5. Double check that all species and charge balance. Under Basic conditions Go all the way to step 4, that is, get the equation completely balanced. 5. Count up the number of H+ ions in the equation. a. Add an equal number of OH- ions to BOTH sides of the equation. b. On the side of the equation where you have both H+ and OH- , these two ions will combine to form H2O. c. On the side where OH is alone, nothing happens. 6. Recheck you equation to see if any H2O may be eliminated from both sides of the equation 7. Double check that all species and charge balance.