The Chemistry of Life PP
advertisement

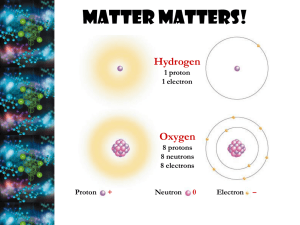
Chapter 2 The smallest particle of an element that has the chemical properties of the element. Three subatomic particles: Protons: + charge Equal mass; Found in Neutrons: no charge the Nucleus Electrons: - charge; in constant movement around the Nucleus # protons = # electrons, so the atom is neutral overall. Amazing Atoms A pure substance that consists entirely of one type of atom. Listed on the periodic table. Ex: hydrogen (H) Contain same number of protons & electrons (so are the same element), but have a differing number of neutrons. Ex: carbon-12 & carbon-14 Two or more elements chemically combined together. The chemical and physical properties of a compound are different than the properties of the individual elements from which it is formed. ▪ Ex: NaCl Two types: Ionic: Electrons are transferred from one atom to another; creates ions ▪ Ions: charged atoms ▪ Ex: Na+, Cl Covalent: Electrons are shared (travel around both nuclei); creates molecules ▪ Molecules: smallest unit of a compound with covalent bonds ▪ Ex: H2O (2 atoms of H, 1 atom of O) A Good Review Composed of two or more elements or compounds. NOT chemically combined. Solution: a mixture with components evenly distributed throughout Two parts: Solute: the part that is dissolved Solvent: the part that is dissolving Acids: form H+ ions in a solution pH = 1-7 Bases: form OH- in a solution pH = 7-14 Buffers: weak acids or bases that react with strong acids or bases to prevent sudden changes in pH Help maintain homeostasis! Carbon has 4 electrons for bonding, so it can form strong covalent bonds with many other elements (like H, O, P, S, and N). Carbon can form single, double and triple bonds with itself. Organic = contains Carbon Monomers: smaller unit Polymers: larger compound Types of Macromolecules: carbohydrates, lipids, nucleic acids, proteins Chemical Composition -C, H, O Examples Function in Living Things - monosaccharides: - Main source of simple sugars energy. ( glucose, fructose, galactose) - Structural purposes in some - polysaccharides: cells (cellulose in complex sugars plants). (glycogen, starch) Chemical Composition Examples Function in Living Things -C, H, O -fats, oil, waxes -Stored energy. - Glycerol + fatty acid -saturated: all single bonds (animal fats = bad!) -Membranes & waterproof coverings. -unsaturated: at least one double bond (vegetable fats = good!) -Chemical messengers (steroids). Chemical Composition -C, H, O, N, P - sugar + phosphate group (P) + nitrogenous base (N) - polymers of nucleotides Examples - DNA, RNA Function in Living Things - Store & transmit genetic information. Chemical Composition -C, H, O, N, S - polymers of amino acids Examples - enzymes Function in Living Things - Control reactions (enzymes) & cell processes. - Transport materials in & out of cells. - Fight disease (antibodies). When one set of chemicals changes into another set of chemicals. CO2 + H2O H2CO3 Chemical reactions always involve the breaking of bonds in the reactants and the formation of new bond in the products. CO2 + H2O H2CO3 Reactants: elements or compounds that enter into a reaction Ex: CO2 & H2O Products: elements or compounds that are produced by a reaction Ex: H2CO3 2HCl + 2Na -> 2NaCl + H2 Remember the 2 after H2, means that there are 2 atoms of H in this molecule. (For H2O, there are 2 atoms of H and 1 atom of O.) The 2 in front of 2HCl, means that there is 2 of the entire molecule, so 2 atoms of H and 2 atoms of Cl. Reactions that release energy often occur spontaneously. Reactions that absorb energy will not occur without a source of energy. Activation Energy: the energy needed to get a reaction started Catalyst: a substance that speeds up the rate of a chemical reaction by lowering the activation energy. Enzymes: proteins that act as catalysts in cells. Substrates: the reactants of an enzymecatalyzed reaction Active site: spot on the enzyme where the substrate binds Video Temperature, pH and regulatory molecules can affect the activity of enzymes. Dehydration synthesis: two molecules bond together & H2O is made Used to make polysaccharides, lipids & proteins Hydrolysis: H2O is used to split two molecules