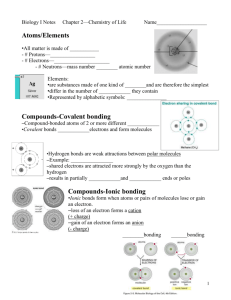
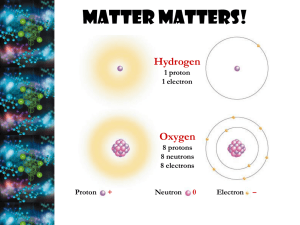
Chemical Basis For Life Why study chemistry in A&P Class?
advertisement

Chemical Basis For Life Why study chemistry in A&P Class? Body functions __________________________ Cellular functions result __________________ Chemistry explains _____________________; and helps _____________________________ Structure of Matter Matter = anything takes up _________________________ Elements= composed of ___________________________ Bulk elements required by body in _________________ Trace Elements requires by body in _______________ Atoms= smallest ______________________ Atomic Structure Protons carry a ___________________________ Neutrons carry a _____________________________ Electrons carry a _____________________________ Nucleus consists of ________________________; central part of the _____________ Electrons move _______________________ Atomic Number = ________________________; each element has a__________________ atomic number Atomic Mass = ________________________ Isotopes atoms with ________________________ but ______________________________________ What changes? Ion an atom that ______________________. What changes? Molecules Particles formed when _______________________________ Molecular formula= ________________ Matter & Elements Why Atoms Form Bonds Sharing electrons Stealing electrons ***Ionic & covalent bond diagrams *** Structural Formula Diagram Polar Molecules & Hydrogen Bonds Molecule with a __________________________________ end Results when electrons are _____________________________ __________ is the most important polar molecule Hydrogen Bond attraction between ___________________ _________________________________ *** Why water is polar diagram Chemical Reactions Chemical reactions occur when _____________________ among atoms, ions, or molecules Reactants are substances _______________________ Products are formed at __________________________ A + B AB Types of Chemical Reactions Synthesis Reaction chemical bonds _______________ ____________________ Decomposition Reaction bonds are _________________ Exchange Reaction chemical bonds are _____________ ____________________ (AB + CD AD + AB) Reversible Reaction The products can _______________ ________________________ Acids + Bases Electrolytes substances that __________________ Acids electrolytes that __________________________ Bases Substances that can _________________________ ________________________________ pH scale 0-6.9= ________ 7 = _______ 7.1-14 = ____________ Organic vs Inorganic Molecules Organic Molecules Contain __________ _________ Dissolve in ___________ _______________________________________ Inorganic Molecules No _________ ________ Dissolve in ___________ ______________________________ Inorganic Examples Water Most abundant ___________________________ _______________ major _________________________ important in ____________________________ Oxygen Used for energy ________________________ CO2 Waste ________________________ Must be __________________________ Salts Abundant _____________________ Sources of _____________________ Important in __________________________ Organic Examples Carbohydrates provide ______________________ help _______________________ monosaccharides= __________________ disaacharides = ______________________ polysaccharides = _______________________ Lipids Fats (triglycerides) Used for __________ Long ___________________ Triglyceride = __________________ Saturated= not easily _________________________ Unsaturated= easily __________________________ Phospholipids are essential to _________________ Steroids = connected rings of carbon used to make ____________________________ Proteins _______________________________ _____________ ______________ ____________ Made from ________________________________ Nucleic Acids Make _______________ Instruct _____________________ Made from ________________ ______________________