Lewis Dot Diagram Practice
advertisement

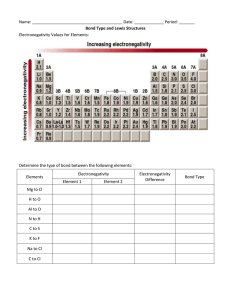
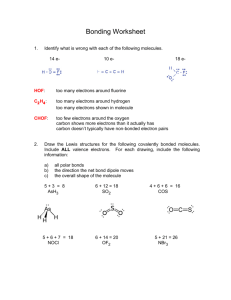
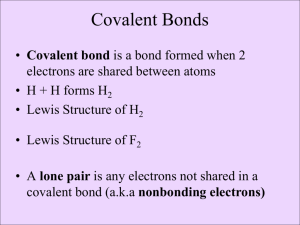
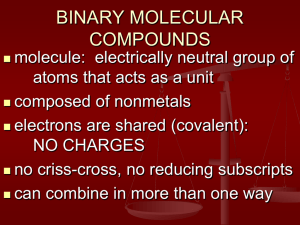
Name: _______________________________________________ Date: _______________ Period: _____ LEWIS DOT PRACTICE Drawing Lewis Structures: 1. Draw skeletal structure of compound showing what atoms are bonded to each other. Put least electronegative element in the center. 2. Count total number of valence e-. Add 1 for each negative charge. Subtract 1 for each positive charge. 3. Complete an octet for all atoms except hydrogen 4. If structure contains too many electrons, form double and triple bonds on central atom as needed. Draw Lewis Structures for the following compounds. 1. F2 6. CO 2. H2O 7. CO32- 3. SCl2 8. SO42- 4. AsF3 9. N2F2 5. SiH4 10. C3H6O Extra Tips: When drawing the initial skeleton, try to make the most symmetrical arrangement possible. Carbon loves to be in the center. Hydrogen and Halogens love to be on the ends and don’t partake in double or triple bonds. Bonds always form between pairs of electrons (you should never see unpaired electrons). Always check the final electron count after you are done drawing! Remember for polyatomic ions to put brackets around the entire structure with the charge written on the outside