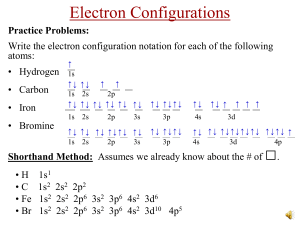
Electron Arrangement Electrons can be found around the nucleus in three-
advertisement

Electron Arrangement Electrons can be found around the nucleus in threedimensional energy levels. Electrons can not be found in between energy levels. They can move from one energy level to the next. The electron’s energy ____________ as it moves to higher energy levels. When the electron moves to a lower energy level it releases energy in the form of _________ (photon). An element’s period number is the same as the number of energy levels that it’s electrons occupy Expressed as: Bohr-Rutherford Diagrams Lewis Structures Ionization – when an atom gains an electron an ________ is formed: Cl + e- Cl1-. There are more electrons than protons. When an atom loses an electron a _________ is formed: Na Na1+ + 1e-. There are fewer electrons than protons. Noble Gases Group VIII elements are the only elements that exist as individual atoms in nature. Extremely unreactive because outer energy level is full. Noble gases have a ____________ octet.