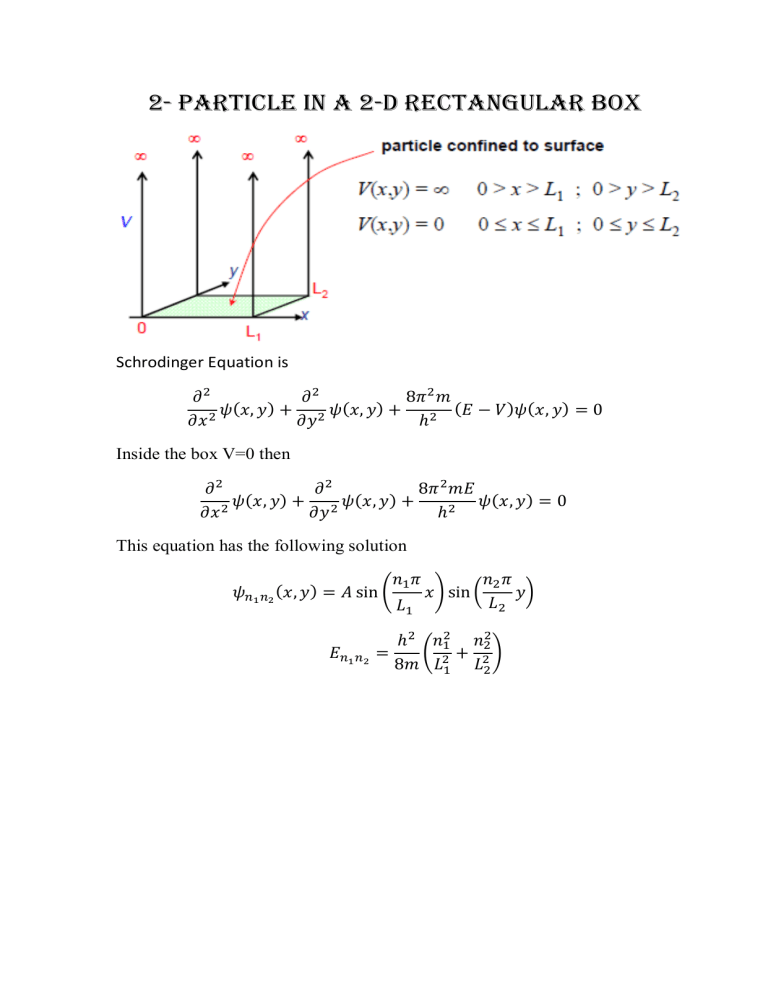
2- Particle in a 2-D Rectangular Box Schrodinger Equation is ( ) ( ) ( ) ( Inside the box V=0 then ( ) ( ) ( ) This equation has the following solution ( ) ( ) ( ( ) ) ) 𝐸𝑛 𝑛 ( 𝑚 𝐿 𝑛 𝑛 ) 𝐿 1 ( 𝑚 𝐿 𝐸 1 ) 𝐿 𝐸 ( 4 𝑚 𝐿 1 ) 𝐿 𝐸 4 𝑚 𝐿 4 ) 𝐿 ( Degeneracy Two (or more) different states with the same energy = Degenerate 𝐸𝑛 𝑛 𝑚 ( 𝑛 𝑛 𝐿⬚ 𝐸𝑛 ) 𝑛 Degenerate states –same energy Different wave functions related by symmetry Degeneracy = 2 Nodal Planes: ( ) 𝑛 ( 𝑚 𝐿 𝑛 ) 𝐿 Examples: Draw the following wave functions and give their energies As a variant on the free-electron model applied to benzene, assume that the six electrons are delocalized within a square plate of side L=4.2 Å. Calculate the 1st π→ π* transition and compare it to experimental value 200 nm. Azulene C10H8, shown below, is an aromatic hydrocarbon containing delocalized π electrons. As a model for this π electron system, consider the mobile electrons in a rectangular two-dimensional box of dimensions 5.00 Å by 4.65 Å. 1- How many π -electrons does azulene have? 2- Identify the quantum numbers of the HOMO and LUMO of the π electron system 3- Calculate the wavelength (in nm) of the lowest-energy π electron transition 4- Should azulene be a colored compound? Naphthalene C10H8, shown below, is an aromatic hydrocarbon containing delocalized π electrons. As a model for this π electron system, consider the mobile electrons in a rectangular two-dimensional box of dimensions 5.60 Å by 4.40 Å. 1- How many π -electrons does naphthalene have? 2- Identify the quantum numbers of the HOMO and LUMO of the π electron system 3- Calculate the wavelength (in nm) of the lowest-energy π electron transition 4- Should naphthalene be a colored compound? 3-Particle in a 3-D Box Schrodinger Equation is ( ) ( ) ( ( ) ( ) ) Inside the box V=0 then ( ) ( ) ( ) ( This equation has the following solution ( ) ( ) ( ( ) ( ) ) ) Degeneracy of Particle in a 3-D Box Two (or more) different states with the same energy = Degenerate ( ) In the Cubic box ( ) The lowest level E111 is non-degenerate but the second level is threefold degenerate The degeneracy occurs in the symmetric cube while in the general rectangular box the degeneracy is destroyed because the symmetry is destroyed by making the sides with different lengths. Examples: Assume that a nucleus can be represented as a cubic box of side 10-14 m. The particles in this box are the nucleons (protons and neutrons). Calculate the lowest allowed energy of a nucleon. Express your result in MeV (1 MeV = 106eV, Mn = 1.67 x 10-27 kg). Consider the hypothetical reaction of two "cube-atoms" to form a "moly-box". Each cube-atom contains one electron. The interaction between electrons can be neglected. Determine the energy change in the reaction. Comment in your results.


