1.7 Trends in the Periodic Table
advertisement

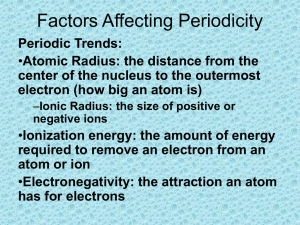
1.7 Trends in the Periodic Table Some trends we already know: • Atoms in the same period have the same number of orbitals / energy levels / shells • Atoms in the same group have the same number of valence electrons 1. Atomic Radius DEFINITION • An atom’s size is measured in terms of its radius. • Distance from the nucleus to the outer energy level. PATTERN • INCREASES down a group • There are more energy levels as you go down a group. Each energy level is a greater distance from the nucleus. • DECREASES across a period • There are more charges – both positive and negative – as you go across a period and therefore a stronger attraction, drawing electrons closer to the nucleus. 2. Ionization Energy DEFINITION • Energy that is required to remove an electron from the atom or ion in its gaseous state. X(g) + energy → X(g)+ + ePATTERN • DECREASES down a group • There are more energy levels as you go down a group, so attraction between valence electrons and the nucleus is weaker, meaning less energy is required to remove a valence electron. • INCREASES across a period • Electrons are held more strongly as you go across a period, making it harder to remove an electron. 2. Ionization Energy **Remember: Metals prefer to give an electron away. Nonmetals do not want to give their electrons away. 1st, 2nd, 3rd IE can be measured (The ability to remove the FIRST, then SECOND, then THIRD electron) The IE takes a much larger jump in value when the next electron to be removed is to be taken from the second outer energy level. Example: Magnesium This shows why atoms will NOT lose electrons from an inner level. It takes too much energy! 3. Electron Affinity DEFINITION • The energy change when an electron is added to a neutral atom X(g) + e- → X(g)- + energy • The amount of energy an atom (or ion) is willing to pay to buy another electron 3. Electron Affinity PATTERN • DECREASES down a group. • There is less attraction for an electron because the nucleus is further away from the outer energy level. • INCREASES across a period. • There is a greater attraction for an electron because the nucleus is closer to the outer energy level. **Remember: Non-metals would prefer to take an electron. Metals do not want electrons. 4. Electronegativity DEFINITION • A measure of an atom’s ability to attract electrons in a chemical bond PATTERN • DECREASES down a group • INCREASES across a period • Fluorine has the highest electronegativity value. most Atomic Radius least Ionization Energy / Electron Affinity / Electronegativity least least most most Please read pages 36-41and answer questions 1-9 on page 41.