Periodic Trends Atomic Radius: Across a period: WHY:
advertisement

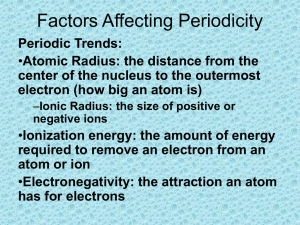
Periodic Trends Atomic Radius: Size of the atom. Across a period: Atomic radius ___________________ moving from left to right. WHY: A proton is added in each element on the same energy level, adding to the power of the nucleus to pull electrons inward. Down a group: Atomic radius ___________________ moving down a group. WHY: Each orbit is overlapped by another larger orbit for each period moving downwards within a group. Ionic Radius: Size of a _______________________. Cation: When a stable cation forms (eg. Na1+) an entire energy level is lost and the ionic radius is _________. Anion: When a stable anion forms (eg. F1-) there are now more electrons than protons. The electron-electron repulsion increases and the nuclear charge remains the same. The anion gets __________. Ionization Energy: The energy required to remove an electron from its outer shell. Across a period: Ionization energy ____________________ moving from left to right across a period. WHY: Increased number of protons increases the energy needed to pull electrons away from the nucleus. More energy is needed for smaller atoms than larger atoms. Down a group: Ionization energy ______________________ moving down a group. WHY: 1) Distance from the positively charged nucleus is greater 2) Shielding effect: Inner electrons block the attraction of the nucleus to the outer electrons. Electron Affinity: Attraction of an atom for an electron. (Electronegativity - Attraction of an atom for an electron within a chemical bond.) Across a period: Electron affinity ______________moving left to right across the periodic table (except group VIII). WHY: Increased number of protons causes increased attraction to other electrons. Smaller atoms have a greater ability to attract an electron. Noble gases have no room on their shell for an electron. Down a group: Electron affinity _____________________ moving down a group. WHY: Larger atom means nucleus is farther from the outer shell and attracts electrons less strongly. Reactivity: Ability of an atom to react. Across a period: Starting from the middle, reactivity _______________________ moving outwards towards the ends of the periodic table, (noble gases not included). WHY: Moving outwards, elements need to give or take fewer electrons to achieve a stable octet Down a group: On the metallic side, reactivity _____________________ moving down the group. WHY: Electrons are so easily removed. Large metals give up electrons easier since they are further from the nucleus. Down a group: On the non-metallic side, reactivity __________________________ moving up the group. WHY: Nucleus has strong attraction to other electrons. Small non-metals gain electrons better and hold electrons closer to its nucleus.
![The electronic configuration of phosphorus is [Ne] 3s2 3p3](http://s3.studylib.net/store/data/008974852_1-8381577ce936fbfa611892c1a5f109cd-300x300.png)