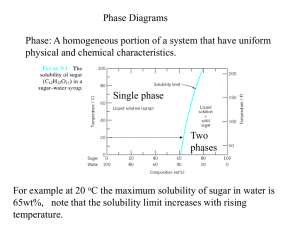
Phase Diagrams
advertisement

Phase Diagrams A phase is a state of matter with the following characteristics: • It has the same structure or atomic arrangement throughout • It has roughly the same composition and properties throughout. • There exists a definite interface between it and its surroundings or adjoining phases. A phase diagram is a graphical representation of the phases that are present in a material at various temperatures and pressures and compositions. • It usually describes the equilibrium conditions • Sometimes non-equilibrium conditions are also shown when well known. • It indicates the melting/solidification temperatures of the constituents • It indicates the compositions of alloys where solidification begins and the temperature range over which it occurs. For a pure substance, the Pressure-Temperature phase diagram simply tells which forms (solid, liquid, gas) of the material exist under different P-T conditions. Phase diagram for water. General Types of Phase Diagrams There are two general types of alloys having phase diagrams. • Substitutional alloys • Interstitial alloys Subtitutional alloys have elements, which are incorporated into regular lattice positions within the unit cell. An example is Tin and Zinc alloying additions to Copper to form bronze and brass, respectively Interstitial alloys have elements, which are incorporated into the interstitial sites of the unit cell. An example is carbon in iron to form steel. interstitial sites of the unit cell. An example is carbon in iron to form steel. Gibb’s Phase Rule Gibb’s phase rule describes the thermodynamic state of a material. This famous rule is used to determine the number of phases that can coexist in equilibrium in a given system. It has the general form: F = C – P + 2 C is the number of components, usually elements or compounds, in the system. F is the number of degrees of freedom, or number of variables, such as temperature, pressure, or composition that are allowed to change independently without changing the number of phases in equilibrium. P is the number of phases present The constant “2” in the equation implies that both temperature and pressure are allowed to change. For the triple point of water: • One component, i.e., water. • 3 phases present, i.e. vapor, liquid, and solid. • F = 1 – 3 + 2 = 0, so this is an invariant point on the diagram Most binary phase diagrams used in materials science are temperature and composition diagrams at a constant 1 atmosphere of pressure. The constant pressure will reduce the degrees of freedom from “2” in Gibb’s equation to “1” for a binary phase diagram Thus, F = C – P + 1.