Day_9 - Rose
advertisement

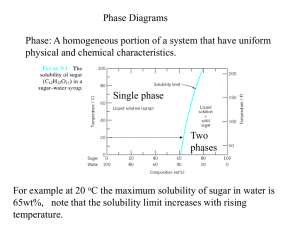
MATERIALS ENGINEERING – DAY 9 PHASES AND PHASE DIAGRAM Preliminary Discussion Concept of the Phase Phase Diagram – start with Copper Nickel. 1. What phase or phases are present? 2. What is the chemical composition of each phase present? 3. How much (relative amounts) of each phase is present? More practice with the Lead Tin system YOU SHOULD BE ABLE TO: Define a phase is and explain how the amount, nature, size, shape, distribution, and orientation of the phases affects the material properties. Given an equilibrium phase diagram, identify the liquidus, solidus, or solvus lines, and state what they represent. identify the eutectic or eutectoid point, composition, or temperature, and state what they represent. find the number of phases present, name those phases, find their chemical compositions (phase analyses), and find in what proportions (amounts) the phases occur. predict whether age hardening is possible for a given alloy. LET’S LOOK AT SOME METALLOGRAPHS An alloy of Cu in Aluminum Al, surrounded by a mixture of Al and CuAl2. Al and CuAl2 mixture. ANOTHER – A CLOSEUP OF A STEEL. The darker area is Fe with small amount of interstitial carbon. The lighter standout areas are the compound cementite, Fe3C. (Iron carbide.) WHAT HAVE WE SEEN? Multiphase materials, or alloys. Phases are separate, they are clearly different materials. But they are mixed together, at times very finely. We do not always have multiphase alloys. There are many useful single phase alloys. BUT The presence of the second phase is very important to… BLOCK DISLOCATIONS! INCREASE STRENGTH. CONCEPT OF THE PHASE Phase: “A distinct state of matter in a system; matter that is identical in chemical composition and physical state and separated.” (Google) Examples 1. Ice in water 2. Sugar and water 3. Cu-Ni system, as a follow on to above. CONCEPT OF EQUILIBRIUM What phases do we get as we cool off a molten metal? This depends very much on the rate of cooling. If we can cool slowly enough, we get phases that are close to what thermodynamicists would call “equilibrium.” This is the basic phase balance that we get if we have enough time and temperature for diffusion to do its work. Diffusion of key species is essential to being able to get equilibrium. PHASE DIAGRAM – A MAP OF THE PHASE OR PHASES PRESENT AS WE CHANGE TEMP AND COMPOSITION Axes: X-Composition. Y-Temperature Web:http ://www.do itpoms.ac .uk/micli b/pds.swf ?targetFr ame=CuNi The symbol a stands for a solid consisting of Ni dissolved in Cu or viscaversa. ANSWERING THE BASIC QUESTIONS- AT B 1. 2. 3. What Phase or Phases are present? What is the composition of each phase? What is the relative amount of each phase 1. Liquid and a 2. CL = 32% Ni and Ca = 43% Ni 3. Use the Lever Rule! THE LEVER RULE – FINDING RELATIVE AMOUNTS Ca Co WL 100% Ca C L 43 35 100% 73% 43 32 Wa 100% WL 27% Once we know how to answer the three major questions, let’s move on to a more complicated binary system. THE LEAD TIN SYSTEM Let’s work on this one together, using the handout.