Inorganic Pharmaceutical Chemistry
advertisement

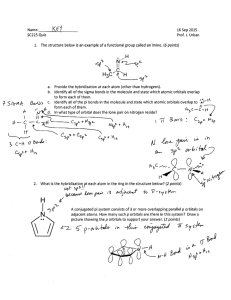
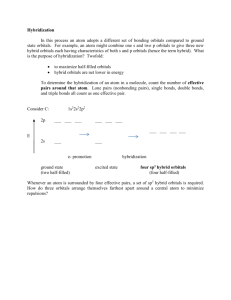
Inorganic Pharmaceutical Chemistry Lecture No. 6 Date : 22/11 /2012 Dr. Mohammed Hamed -------------------------------------------------------------------------------------------------------------------------------------- Hybrid Orbitals Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to 109o, 120o, or 180o. According to Valence Shell Electron Pair Repulsion (VSEPR) theory, electron pairs repel each other and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. Based on the valence bond theory, carbon would only be able to form two covalent bonds, making CH2. However, as you will find out, we know that this is not true and that in reality, it makes CH4. The hybridization of orbitals is also greatly favored because hybridized orbitals are lower in energy compared to their separated, unhybridized counterparts. This results in more stable compounds when hybridization occurs. Also, major parts of the hybridized orbitals, or the frontal lobes, overlap better than the lobes of unhybridized orbitals. This leads to better bonding. Carbon is a perfect example showing the need for hybrid orbitals. As you know, Carbon's ground state configuration is: 6 C 1S2 2S2 2P2 According to the valence bond theory, carbon should form two covalent bonds, resulting in a CH2. However, tests show that CH2 is highly reactive and cannot exist outside of a reaction. Therefore, this does not explain how CH4 can exist. However, you can excite a 2s electron and bump it into one of the 2p orbitals. This would give you the following configuration: While this would allow us to have four covalent bonds, resulting in CH4, it also implies that the C-H covalent bonds would have different energies due to the different levels of orbital overlap. However, with testing, it has been proven that in CH4, any hydrogen can be removed with the same amount of energy. This means that every C-H covalent bond should have equal energies. Once again, this means that the valence bond theory fails to explain the existence of CH 4. The only way it can be explained is if when we had the exited state above, the 2s and the 3 2p orbitals fused together to make four, equal energy sp3 hybrid orbitals. That would give us the following configuration: Z Z Y 2S X Y 2PX X Y Z 2Py Z X C Y 2PZ X This explains how a carbon can have four equal energy bonds. The next section will explain the various types of hybridization and how each type helps explain the structure of certain molecules. TYPES OF HYBRIDIZATION During hybridization, the atomic orbitals with different characteristics are mixed with each other. Hence there is no meaning of hybridization between same type of orbitals i.e., mixing of two 's' orbitals or two 'p' orbitals is not called hybridization. However orbital of 's' type can mix with the orbitals of 'p' type or of 'd' type. Based on the type and number of orbitals, the hybridization can be subdivided into following types. sp HYBRIDIZATION * Intermixing of one 's' and one 'p' orbitals of almost equal energy to give two identical and degenerate hybrid orbitals is called 'sp' hybridization. * These sp-hybrid orbitals are arranged linearly at by making 180o of angle. * They possess 50% 's' and 50% 'p' character. sp2 HYBRIDIZATION * Intermixing of one 's' and two 'p' orbitals of almost equal energy to give three identical and degenerate hybrid orbitals is known as sp2 hybridization. * The three sp2 hybrid orbitals are oriented in trigonal planar symmetry at angles of 120o to each other. * The sp2 hybrid orbitals have 33.3% 's' character and 66.6% 'p' character. sp3 HYBRIDIZATION * In sp3 hybridization, one 's' and three 'p' orbitals of almost equal energy intermix to give four identical and degenerate hybrid orbitals. * These four sp3 hybrid orbitals are oriented in tetrahedral symmetry with 109o28' angle with each other. * The sp3 hybrid orbitals have 25% ‘s’ character and 75% 'p' character. sp3d HYBRIDIZATION * In sp3d hybridization, one 's', three 'p' and one 'd' orbitals of almost equal energy intermix to give five identical and degenerate hybrid orbitals, which are arranged in trigonal bipyramidal symmetry. Among them, three are arranged in trigonal plane and the remaining two orbitals are present above and below the trigonal plane at right angles. The sp3d hybrid orbitals have 20% 's', 60% 'p' and 20% 'd' characters. sp3d2 HYBRIDIZATION * Intermixing of one 's', three 'p' and two 'd' orbitals of almost same energy by giving six identical and degenerate hybrid orbitals is called sp3d2 hybridization. * These six sp3d2 orbitals are arranged in octahedral symmetry by making 90 o angles to each other. This arrangement can be visualized as four orbitals arranged in a square plane and the remaining two are oriented above and below this plane perpendicularly. sp3d3 HYBRIDIZATION * In sp3d3 hybridization, one 's', three 'p' and three 'd' orbitals of almost same energy intermix to give seven sp3d3 hybrid orbitals, which are oriented in pentagonal bipyramidal symmetry. * Five among the sp3d3 orbitals are arranged in a pentagonal plane by making 72o of angles. The remaining are arranged perpendicularly above and below this pentagonal plane. In 1927, valence bond theory was formulated and it argues that a chemical bond forms when two valence electrons, in their respective atomic orbitals, work or function to hold two nuclei together, by virtue of effects of lowering system energies. Pauling presented six rules for the shared electron bond, 1. The electron-pair bond forms through the interaction of an unpaired electron on each of two atoms. 2. The spins of the electrons have to be opposed. 3. Once paired, the two electrons cannot take part in additional bonds. 4. The electron-exchange terms for the bond involves only one wave function from each atom. 5. The available electrons in the lowest energy level form the strongest bonds. 6. Of two orbitals in an atom, the one that can overlap the most with an orbital from another atom will form the strongest bond, and this bond will tend to lie in the direction of the concentrated orbital. THE SHAPES OF COMPLEX METAL IONS To describes the shapes of some complex metal ions. It goes on to look at some simple examples of stereoisomerism (geometric and optical) in complex ions. These shapes are for complex ions formed using monodentate ligands - ligands which only form one bond to the central metal ion. 6-coordinated complex ions These are complex ions in which the central metal ion is forming six bonds. In the simple cases we are talking about, that means that it will be attached to six ligands. These ions have an octahedral shape. Four of the ligands are in one plane, with the fifth one above the plane, and the sixth one below the plane. The diagram shows four fairly random examples of octahedral ions. 4-coordinated complex ions These are far less common, and they can take up one of two different shapes. Tetrahedral ions There are two very similar ions which crop up commonly at this level: [CuCl 4]2and [CoCl4]2-. The copper(II) and cobalt(II) ions have four chloride ions bonded to them rather than six, because the chloride ions are too big to fit any more around the central metal ion A square planar complex Occasionally a 4-co-ordinated complex turns out to be square planar. There's no easy way of predicting that this is going to happen. The only one you might possibly come across at this level is cisplatin which is used as an anti-cancer drug. Cisplatin is a neutral complex, Pt(NH3)2Cl2. It is neutral because the 2+ charge of the original platinum(II) ion is exactly cancelled by the two negative charges supplied by the chloride ions The platinum, the two chlorines, and the two nitrogens are all in the same plane. We will have more to say about cisplatin immediately below Stereoisomerism in complex ions Some complex ions can show either optical or geometric isomerism. Geometric isomerism This occurs in planar complexes like the Pt(NH3)2Cl2 we've just looked at. There are two completely different ways in which the ammonias and chloride ions could arrange themselves around the central platinum ion: The two structures drawn are isomers because there is no way that you can just twist one to turn it into the other. The complexes are both locked into their current forms The terms cis and trans are used in the same way as they are in organic chemistry. Trans implies "opposite" - notice that the ammonias are arranged opposite each other in that version, and so are the chlorines. Cis implies "on the same side" - in this instance, that just means that the ammonias and the chlorines are next door to each other. Optical isomerism You recognise optical isomers because they have no plane of symmetry. In the organic case, it is fairly easy to recognise the possibiliy of this by looking for a carbon atom with four different things attached to it. It isn't qute so easy with the complex ions - either to draw or to visualise! The examples you are most likely to need occur in octahedral complexes which contain bidentate ligands - ions like [Ni(NH2CH2CH2NH2)3]2+ or [Cr(C2O4)3]3-. The diagram below shows a simplified view of one of these ions. Essentially, they all have the same shape - all that differs is the nature of the "headphones". I have deliberately left the charges off the ion, because obviously they will vary from case to case. The shape shown applies to any ion of this kind. If your visual imagination will cope, you may be able to see that this ion has no plane of symmetry. If you find this difficult to visualise, the only solution is to make the ion out of a lump of plasticene (or a bit of clay or dough) and three bits of cardboard cut to shape A substance with no plane of symmetry is going to have optical isomers - one of which is the mirror image of the other. One of the isomers will rotate the plane of polarisation of plane polarised light clockwise; the other rotates it anticlockwise Coordination complexes in biochemistry Approximately one-third of the chemical elements are present in living organisms. Many of these are metallic ions whose function within the cell depends on the formation of d-orbital coordination complexes with small molecules such as porphyrins (see below). These complexes are themselves bound within proteins (metalloproteins) which provide a local environment that is essential for their function, which is either to transport or store diatomic molecule (oxygen or nitric oxide), to transfer electrons in oxidation-reduction processes, or to catalyze a chemical reaction. The most common of these utilize complexes of Fe and Mg, but other micronutrient metals including Cu, Mn, Mo, Ni, Se, and Zn are also important. Geometry and magnetic nature of some complexes Type No. of Geome Oxidation Magnetic of unpaired try state of nature hybrid electrons shape metal (7) ization (6) (5) (3) (4) Paramag netic Paramag netic Diamagn etic Paramag netic Diamagn etic Ni2+(d8) +2 [NiCl4]2– +2 [Ni(CN)4]2+ 0 Ni 0 Ni(CO)4 +2 [Ni(NH3)6]2 +2 Mn2+(d5) 1 Octah d2sp3(I edral nner) +2 [Mn(CN)6]4– 5 Tetrah sp3 edral +2 [MnCl4]2 +2 Cu2+(d9) +2 [CuCl4]2– +2 [Cu(NH3)4]2 2 0 Tetrah sp3 edral Squar e dsp2 planar 2 Tetrah sp3 edral Paramag netic 2 Octah sp3d2(out edral er) Paramag netic 5 Paramag netic Paramag netic Paramag netic Paramag netic Atom/ion/ complex (1) +2 2 0 Paramag netic Configuration (2) 1 1 1 Tetrah sp3 edral Squar dsp2 e planar + + Paramagn etic 3 Paramagn etic 3 Paramagn etic 3 Paramagn etic 4 Paramagn etic 4 Diamagnet ic 0 Paramagn etic 3 Paramagn etic Paramagn etic Diamagnet ic Paramagn etic Paramagn etic Paramagn etic 3 Cr3+(d3) Octahe d2sp3 (I dral nner) +3 [Cr(NH3)6]3+ Octahe sp3d2 ( dral Outer) +3 [Cr(H2O)6]3+ +3 CO3+(d6) Octahe sp3d2 ( dral Outer) +3 [CoF6]3– Octahe d2sp3 (I dral nner) +3 [Co(NH3)6]3+ +2 CO2+(d7) +2 [Co(H2O)6]2+ +2 Fe2+(d6) Octahe sp3d2 ( dral Outer) 4 0 Octahe d2sp3 (I dral nner) +2 [Fe(CN)6]4– 4 Octahe sp3d2 ( dral Outer) +2 [Fe(H2O)6]2+ 4 5 Paramagn etic 1 Paramagn etic 4 Diamagnet ic +3 o Octahe sp3d2 ( dral Outer) Octahe dral d2sp3 (I nner) Trigon al dsp3 bipyra (Inner) midal +2 Same [Fe(NH3)6]2+ +3 Fe3+(d5) +3 [Fe(CN)63– o Fe o Fe(CO)5