Balancing Equations
advertisement
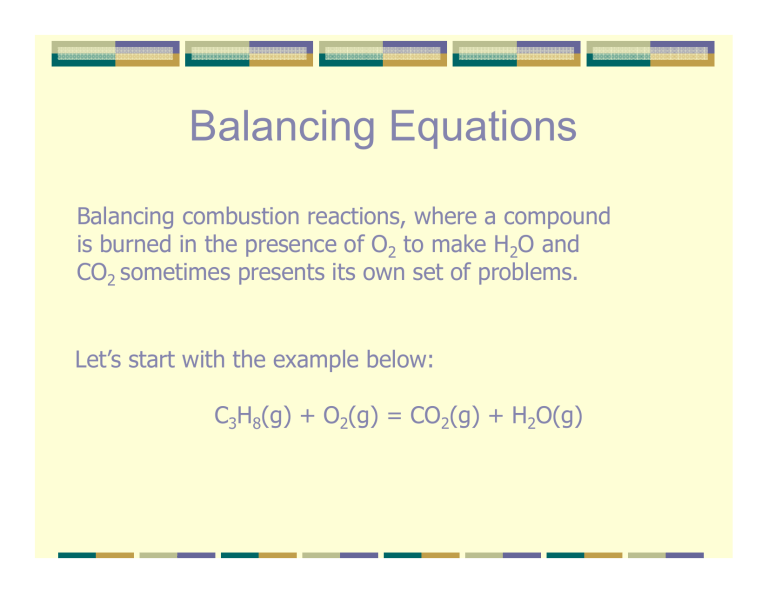
Balancing Equations Balancing combustion reactions, where a compound is burned in the presence of O2 to make H2O and CO2 sometimes presents its own set of problems. Let’s start with the example below: C3H8(g) ( ) + O2(g) ( ) = CO2(g) ( ) + H2O(g) O( ) ? C3H8(g) + ? O2(g) = ? CO2(g) + ? H2O(g) C 3 0 1 0 In combustions reactions, dealing with the O is tricky because there are two different products that contain O. As a result I always save O for last when I balance a combustion reaction. Therefore let’s start with the C. We have 3 C’s on the left, so we will need 3 CO2’s on the th right. i ht 1 C3H8(g) + ? O2(g) = 3 CO2(g) + ? H2O(g) C H 1(3) 1(8) ( ) 0 0 3(1) 0 0 2 Since we are saving O for last, that makes H next. We have 8 H’s on the left, so we will need 4 H2O’s on the th right. i ht 1 C3H8(g) + ? O2(g) = 3 CO2(g) + 4 H2O(g) C H O 1(3) 1(8) ( ) 0 0 0 2 3(1) 0 3(2) 0 4(2) ( ) 4(1) And finally the O’s. We have a total of 6+ 4 O’s on the left, so we will need 10 O’s O’ on the th right, i ht or 5 O2’s. ’ 1 C3H8(g) + 5 O2(g) = 3 CO2(g) + 4 H2O(g) C H O 1(3) 1(8) ( ) 0 0 0 5(2) 3(1) 0 3(2) 0 4(2) ( ) 4(1) Well that wasn’t so bad. Our final answer is: 1 C3H8(g) + 5 O2(g) = 3 CO2(g) + 4 H2O(g) That was nice and easy. g a little harder. Now let’s do something This is a similar combustion that is a little trickier: ? C4H10(g) + ? O2(g) = ? CO2(g) + ? H2O(g) C 4 0 1 0 As before, start with C. 4 C’s on the left,, so we need 4 CO2’s on the right. g 1 C4H10(g) + ? O2(g) = 4 CO2(g) + ? H2O(g) C 1(4) 0 4(1) 0 H 1(10) 0 0 2 Next move on to H’s. 10 H’s on the left,, so we need 5 H2O’s on the right. g 1 C4H10(g) + ? O2(g) = 4 CO2(g) + 5 H2O(g) C 1(4) 0 4(1) 0 H 1(10) 0 0 5(2) O 0 2 4(2) 5(1) Last, but b not least, l the h O’s ’ There are 8 + 5 or 13 O’s on the right, so we need 13 O’s on the h left. l f But how h do d you get O2 to give you 13 O’s? ’ What we will do is a cross-multiplication. p We will multiply py anything on the right side of the equation that has O’s by the number of O’s on the left side, and multiply anything that has O’s O s on the left hand hand, by the number of O’s O s on the right. 1 C4H10(g) + ? O2(g) = 4 CO2(g) + 5 H2O(g) O 0 2 2 O’s in 1O2 O 0 4(2) 5(1) 13 O’s total this side Cross-multiplication Cross multiplication 1(2) 4(2) [[1(2)]x13 ( )] 3 [[4(2) ( ) 5(1) 5( 5(1)]x2 )] After the cross multiplication p our equation q looks like this: ? C4H10(g) + 13 O2(g) = 8 CO2(g) + 10 H2O(g) (g). Once you y Notice how I stuck a ? In front of the C4H10(g) have done a cross multiplication, you have to start all over! That’s what we’ll do next. ? C4H10(g) + 13 O2(g) = 8 CO2(g) + 10 H2O(g) O C 0 4 13(2) 0 8(2) 8(1) 10(1) 0 Now we have to start all over with the C’s 8C C’ss on the right right, so we need two molecules of C4H10(g) 2 C4H10(g) + 13 O2(g) = 8 CO2(g) + 10 H2O(g) O C H 0 2(4) 2(10) 13(2) 0 0 8(2) 8(1) 0 10(1) 0 10(2) Now try the H’s again Hooray, it finally worked. Our final answer is: 2 C4H10(g) + 13 O2(g) = 8 CO2(g) + 10 H2O(g) (g) Now try the last balancing tutorial to see where cross-multiplication is used in other problems.







