CHEM 1211K Homework Chapter 3 Name: Student ID: Balance the
advertisement

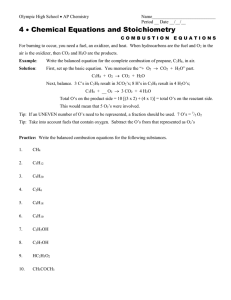
CHEM 1211K Homework Name: Chapter 3 Student ID: 1. Balance the following equations: a. Al(OH)3 (s) + H2SO4 (aq) → Al2(SO4)3 (aq) + H2O (l) b. C5H10 (l) + O2 (g) → CO2 (g) + H2O (l) 2. Determine the molecular formula for a compound that is 70.79% carbon, 8.91% hydrogen, 4.59% nitrogen, and 15.72% oxygen. 3. Determine the molecular formula of a compound that has a molar mass of 92.0 g/mol and an empirical formula of NO2. 4. How many atoms of oxygen are contained in 47.6 g of Al2(CO3)3? The molar mass of Al2(CO3)3 is 233.99 g/mol. 5. Lead (II) carbonate decomposes to give lead (II) oxide and carbon dioxide: PbCO3 (s) → PbO (s) + CO2 (g) How many grams of lead (II) oxide will be produced by the decomposition of 7.50 g of lead (II) carbonate? 6. How many moles of N2O3 contain 2.55 × 1024 oxygen atoms? 7. What is the mass of 9.44 × 1024 molecules of NO2? The molar mass of NO2 is 46.01 g/mol. 8. Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO (s) + H2O (l) → Ca(OH)2 (s) In a particular experiment, a 1.50-g sample of CaO is reacted with excess water and 1.48 g of Ca(OH)2 is recovered. What is the percent yield in this experiment? 9. If 2352 grams of FeS2 is allowed to react with 1408 grams of O2 according to the following equation, how many grams of Fe2O3 are produced? FeS2 + O2 → Fe2O3 + SO2 10. The combustion of propane (C3H8 ) in the presence of excess oxygen yields CO2 and H2O: C3H8 (g) + 5O2 (g) → 3CO2 (g) + 4 H2O (g) When 7.3 g of C3H8 burns in the presence of excess O2, How much g of CO2 is produced?