PowerPoint - Orbital Shape & Orientation
advertisement

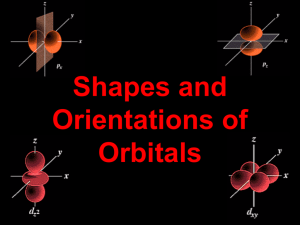
Shapes and Orientations of Orbitals Periodic table arrangement s (n) d (n - 1) p (n) 1 2 3 4 5 6 7 f (n -2) • the quantum theory helps to explain the structure of the periodic table. • n - 1 indicates that the d subshell in period 4 actually starts at 3 (4 - 1 = 3). • The location of electrons is described by: n, l, ml n = size, l = shape, ml = orientation Electron clouds • The “probability” of finding an electron around a nucleus can be calculated. • Relative probability is indicated by a series of dots, indicating the “electron cloud”. • 90% electron probability/cloud for 1s orbital (notice higher probability toward the centre) p orbitals and d orbitals p orbitals look like a dumbell with 3 orientations: px, py, pz (“p sub z”). Four of the d orbitals resemble two dumbells in a clover shape. The last d orbital resembles a p orbital with a donut wrapped around the middle. n 1 l 0(s) ml 0 2 0(s) 1(p) 3 0(s) 1(p) 2(d) 0 -1, 0, 1 0 -1, 0, 1 -2,-1, 0,1, 2 0 4 0(s) ms E N E R G Y 3d 4s 3p 3s 2p 2s 1s Movie: periodic table of the elements: t10-20
![6) cobalt [Ar] 4s 2 3d 7](http://s2.studylib.net/store/data/009918562_1-1950b3428f2f6bf78209e86f923b4abf-300x300.png)