Redox-Active Ligands Q. Michaudel Baran Lab GM 2013-10-19
advertisement

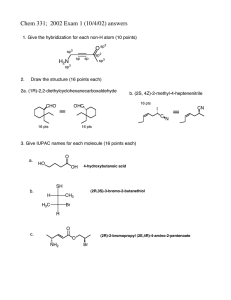
Redox-Active Ligands Q. Michaudel "Although we loved these ligands, by the end of the 1960s, we knew that they were “guilty” as charged." Introduction: the Wacker example and non-innocent ligands H. B. Gray, Inorg. Chem., 2011, 50, 974. OH O2 Pd(II) Ph Baran Lab GM 2013-10-19 Modern experimental (EPR, Raman, X-ray crystallography...) and computational methods can often identify the appropriate oxidation state of the metal and the ligand. Biological relevance: Several enzymes contain non-innocent ligands like molybdenum oxotransferases (see Michaudel Group Meeting 2012) HO O O Ph O Me + Pd(0) Ph HN H2O CHO molybdopterin O Pd N N OR R = Me, H N N Inorg. Chim. Acta, 2011, 370, 374. Appending quinone moieties into a palladium complex could improve the rate of the reaction by proximity effect. However, both NHC complexes (R = Me or H) failed to catalyze oxidation of styrene, as well as the oxidized (quinone) version. Introducing a redox-active substructure in a ligand is not that simple! O + e– O O – e– O – e– O Dithiolene: S II S semiquinone O O L S S' M+n L–2 S' M+(n-2) L low energy M high energy M oxidation state oxidation state (analogous to releasing electrons by the ligand) S X M L S S M+(n+1) L induce radical-type reactivity on the substrate-ligand 2 – e– S II S – e– S Ni S P increased Lewis acidity on the metal– e– ligand involved in substrate S X (analogous for basicity) bond making/breaking spectator ligand M+n L actor ligand catecholate Ni S O O N O H H Four Different Categories of Redox-Active Ligands : Non-Innocent ("Suspect") Ligands (Jørgensen, 1966): Ligands that have an ambiguous oxidation state: NO+/NO /NO– or O2/O2 –/O22– + e– S For two recent and exhaustive reviews, see Crabtree, Chem. Soc. Rev., 2013, 42, 1440; de Bruin, ACS Catal., 2012, 2, 270. M+n O S Redox-active ligands recently regained an increasing attention from the inorganic community and have been the topic of a recent Forum issue in Inorganic Chemistry (Chirik, Inorg. Chem., 2011, 50 (20), 9737–9914.) and a special issue in the European Journal of Inorganic Chemistry (de Bruin, Eur. J. Inorg. Chem., 2012, 3, 340–580.) Br Br orthoquinone N RO OR RO H2N H H N + e– S II S Ni S + e– S S The complex is better described by radical ligands than oxidation of Ni(II) to Ni(IV) This group meeting will not discuss: * electrochemical recognition of cations or anions by a ligand. * redox-switchable hemilabile ligands or reactive fragment redox-switchable ligands. (For a review, see: Mirkin, Angew. Chem., Int. Ed., 1998, 37, 894). 1 Redox-Active Ligands Q. Michaudel Baran Lab GM 2013-10-19 Biomimetic Alcohol Oxidation Actor Ligands: R RO O II S II Cu Cu S O OR R Cooperative Ligand-Centered Reactivity: Mechanism of Galactose-Oxidase O2 Tyr495 2 R'2CHOH Wieghardt, Angew. Chem., Int. Ed., 1998, 37, 2217. R' H2O2 His591 O N His694 NH II H2O H GAO RCH2OH + O2 RCHO + H2O2 Tyr272 N Cu O HN Net reaction: S Cys228 Inactive HO Tyr Tyr O Active RCH2OH II H2O N R' R' H2O O H R R' R' RH II H RCH2OH S N Cu O air, r.t., 12 h S N O air, r.t., 12 h O2 HO R OH R R R R = Me, 61% R = Ph, 68% First biomimetic complexes of Galactose Oxidase (various R and R' groups). Only oxidizes activated alcohols (benzylic or allylic). See Stack, J. Am. Chem. Soc. 1996, 118, 13097 and Science 1998, 279, 537. N I N Cu O H H S N S N Cu R PCET: Proton Coupled Electron Transfer tBu Tyr II O tBu HO N Cu O O tBu O II O II S Cu Cu S O O tBu R = Me, 63% R = Ph, 60% RCHO R2CHOH Tyr HO PCET tBu tBu H R RCHO Tyr H cat. (0.1 mol%) H O S H2O N 2 tBu N Cu O cat. (0.1 mol%) HO tBu RO II O II SH Cu Cu S O OR OH H2O2 II R RO II O II S Cu Cu S O OR R Primary alcohols are oxidized to aldehydes with the same system. N PCET N Cu O S R' HO II N Cu O R' R' Tyr HO N H OR H O S RO O II II Cu Cu S O OR R H H O RH O – e– HO R' R' H R O RO S II II Cu Cu S O OR R H R' R' Que, Nature 2008, 455, 333. O R' R O R' 2 Redox-Active Ligands Q. Michaudel Baran Lab GM 2013-10-19 HQ2– + RCHO KOtBu M = Cu or Zn SQ N tBu N tBuOH N I Ir tBu II H2O2 HN M O O O2 + RCH2OH tBu tBu HN I N N Ir – RCH2OH I N Ir O H BQ N tBu N N tBu tBu II N SQ M M O H R tBu II O O H tBu tBu tBu e– transfer (fast) O I H H O N II N R RCH2OH tBu M O O HOH tBu R tBu Wieghardt, J. Am. Chem. Soc. 1999, 121, 9599 Substrate H EPR spectroscopy, magnetic and electrochemical data provides evidence of ligand participation. It is also of note that Zn2+ is d10 and cannot accommodate any redox catalysis. Catalyst is more efficient with Cu than with Zn and even MeOH can be oxidized (>95%). The active catalyst was prepared by oxidation with ferrocenium: [M(L)] + [Fc]PF6 [M(L')]PF6 + Fc Cat. (0.01 mol%) KOtBu (0.03 mol%) BQ (1.2 equiv.) O OH O > 98% (3 h) N Substrate 94% (3 min) OH OH Me N CH3OH Angew. Chem., Int. Ed., 2007, 46, 3567. CH2O Yield (Time) Product OH O 64% (4 h) OH Me O OH O OH Me O HN I Ir N NH2 BQ = Benzoquinone, SQ = Semiquinone, HQ2– = Hydroquinone CH3CH2OH CH3CHO 94% (4 h) > 98% (2 h) OH O Me N (gram scale, but GC/NMR yield) RCHO PhCl, 80 °C, Ar Yield (Time) Product I RCH2OK H r.d.s. N Ir RCHO tBu N N Ir tBu H R N O H OH OH O > 98% (1 h) > 98% (10 min) BQ (2 equiv.) > 98% (5 min) BQ (2 equiv.) 3 Redox-Active Ligands Q. Michaudel Mechanism of Water Oxidation with Ru "Blue Dimer": Redox-Active Ligand or Not? 4 O V L2Ru H O O 4 V O IV RuL2 L2Ru O O O H O2 Purification of Ethene Gas Streams: IV O III L2Ru OH H2O III F3C S 4 O V V L2Ru O N O H O O IV IV Ru L2Ru OH N H O H H O H HO HO S Ph2P III L2Ru H2O N H2O O2 O S F3C S II S CF3 S CF3 Ni Science 2001, 291, 106. MCS = Multi-Component Stream H2C S III Re S Ph2P Ph [C] + e– III F3C H 4 Ru CF3 CH2 P L N S Improved system: Fixation of ethene faster with oxidized complex [C]2+ and release requires less energy with to reduce complex [D]2+ into [D]. 4 H2O CH2 + MCS IV Ph O H2C Ru Hurst, Inorg. Chem., 2008, 47, 1753. III CF3 MCS electrochemical – e reduction L N OH N HO H2C H2O L2Ru S H2C CH2 4 L N O CF3 CF3 N 4 Ru S F3C N V S S Ni L= L2Ru II II S F3C OH2 H2O S Ni electrochemical oxidation RuL2 Meyer, J. Am. Chem. Soc., 2000, 122, 8464. L N F3C e– 4 RuL2 H Baran Lab GM 2013-10-19 CH2 S Ph2P P K1 Ph – e– S Re S Ph2P Ph [D] + e– – e– L N III Ru H2C H2O N [D]+ K2 H O O H + e– – e– + e– H2C The original Meyer mechanism is still regarded as more likely, but the Hurst mechanism is still under consideration. CH2 [C]+ – e– CH2 [D]2+ [C]2+ K3 J. Am. Chem. Soc., 2009, 131, 64. K3 > K2 > K1 4 Redox-Active Ligands Q. Michaudel Baran Lab GM 2013-10-19 Cyclopropanation with Redox Non-Innocent Carbene: Cooperative Substrate-Centered Radical-Type Reactivity: R' Carbenoid Redox-Active Actor Ligand: MII, d7 RO2C M M H R R H N py py dπ dπ MII N N N III Co N CO2R 2 H R' N N2 N III Co N 4 N CO2R H Electronic configuration of metalloradicals of group 9 N II Co N N π MIII M = Rh, Ir, Co H π* (SOMO) + Chem. Soc. Rev., 2013, 42, 1440 N2 N R' RO2C dz2 dz2 CO2R N II N Co N N MIII, d6 N J. Am. Chem. Soc., 2010, 132, 10891. C–H Amination of Benzylic C–H Bonds: N Me II Ir N N CO2Et Me Me N2 MeCN – H2C 2 N N Me Me MeO2C III Ir N N N N N E N N III Co N N MeO2C H Ar Me Chem.–Eur. J., 2008, 14, 7594. Me H Reactivity of radical carbenoid is different from both Fischer (electrophilic, carboncentered LUMO) and Schrock (nucleophilic, carbon-centered HOMO) carbenes. Ph Me (10 equiv.) N3 N NH Me III Ir N N II Co N O Ar Me Me N Me MeO N Me N N N H MeO III Ir N Me EtO2C Me CH2 Ar O Me N N N E = CO2Me N N III Co N N2 N J. Am. Chem. Soc., 2011, 133, 12264. TrocN3 (1 equiv.) Co(TPP) (5 mol %) 40 °C, 48 h, N2, 69% II Co N N N N N N NHTroc Ph Me Organometallics 2010, 29, 389. 5 Redox-Active Ligands Q. Michaudel Another C–H Amination System: Intermolecular Version: Baran Lab GM 2013-10-19 R3 Mes R1 N II H Betley, J. Am. Chem. Soc., 2011, 133, 4917. N Fe Ph H N3 N3 R2 N3 Ph Product Boc N + PhMe (solvent) Ph 60 °C, 12 h, N2 N H H Me N3 H N3 R 23 °C, benzene N II Fe N Me N3 Boc2O 65 °C, 12 h Cl Fe H H R N3 Me 17% N3 E (1 equiv.) Boc2O (1 equiv.) Boc N H N II Fe N Me H R radical rebound Boc N N3 Me CoII(ttp) (5 mol%) KOH (10 equiv.) t I BuOH (10 equiv.) PhH (100 equiv.) Me Boc Me N Me same conditions C–H Arylation of Benzene: Cl NH R 67% (45%) Me Me Fe BocHN benzene, 23 °C, 12 h same conditions N Me Me H N3 III OTMS Boc Me S N 75% Ph 93%ee H Me Cl 47% 68% OTMS N3 Boc N Yield N3 Ph R Boc Me N Me N2 E H Product Boc N O Boc N 49% Me III N Cl OEt2 R Me H H H H H H Me Betley, Science 2013, 340, 591. Cl H Ph N3 72% Boc N Me O Ph H H Intramolecular Version: Cl Boc N N3 R4 Substrate H [Fe] R2 R3 Yield 57% Boc R5 N R6 R1 benzene, 65 °C, 12 h R4 Substrate Et2O Cl E (10–20 mol%) Boc2O (1 equiv.) R5 R6 N2, dark, 200 °C, Me 3.5 h, 88% (82%) tBu Me Me Eur. J. Inorg. Chem. 2012, 485. HBoc N Me (47%) Me 1.0:1.5 CoII(ttp) Me Ph CoIII(ttp) Me ttp = tetratolylporphyrin 6 Redox-Active Ligands Q. Michaudel Spectator Ligands: Baran Lab GM 2013-10-19 Redox Switch Polymerization: N O Modification of the Lewis Acid-Base Properties of the Metal H2 oxidation: Cp* III III AgBF4 N F3C tBu Ir Cp*2Fe N O F3C tBu Ag A tBu tBu Cp* [Ox + e–] + 2H+ III Ir [Ox] + 2B Ox = AgBF4 or B O tBu AgOTf N O B tBu Fe N O Ti OiPr tBu Fe N TBP F3C tBu tBu tBu N Me tBu A (0.33 equiv.) AgBF4 (2 equiv.) TBP (2 equiv.) J. Am. Chem. Soc., 2008, 130, 788. Fe OiPr increased Ir Lewis acidity H2 H2 N O Ti OiPr tBu Fe Cp* Ir O OiPr O O H2 2 O O P Co I RhSn P Ph Ph P Co – I RhSn e– H2 P Ph Ph increased phosphine Lewis basicity S = Me2CO J. Am. Chem. Soc., 2006, 128, 7410. Me Me Ph Ph + e– n Me or / Ph Ph O Redox-switches could be useful for block copolymerization. Hydrogenation / Me O cat. H+ 1.5 h, r.t. 2 O OH or Me Ph2P Me N J. Am. Chem. Soc., 1995,117, 3617. Cobaltocene reduction enhances electron density on Rh, which facilitates the oxidative addition of H2 (r.d.s.) and leads to 16-fold rate increase compared to the oxidized form of the complex. Interestingly, the oxidized complex catalyzes the hydrosilylation of alkene faster than the reduced form. Fe Ph2P tBu O M N OR Ph2P O FcBArF CoCp2 tBu tBu N O M Fe N OR Ph2P J. Am. Chem. Soc., 2011, 133, 9278 O BArF tBu For M = Y, R = tBu, ferrocenium stops lactide polymerization. While, for M = In, R = Ph, it is the opposite behavior! R.d.s. is different depending on which complex is used. 7 Redox-Active Ligands Q. Michaudel Redox Non-Innocent Ligands as Electron-Reservoirs: Redox-Active Ligands Confer Nobility on Base-Metal Catalysts: Baran Lab GM 2013-10-19 Formal [2 + 2] cycloadditions: Precious metals most efficiently catalyze two electron reactions, since they typically undergo ±2 e–oxidation state changes (IrI, IrIII, IrV or Pd0, PdII, PdIV) whereas basemetal undergo ±1 e–oxidation state changes (CoI, CoII, CoIII or FeII, FeIII). However, the price of precious metals is increasing due to their low abundance. Me iPr Me N II N Fe N2 iPr N N2 iPr F reduced ligand, FeII iPr Abundance in Earth's crust (ppb by weight) X Me Me N N II Fe Me N Me N N Ar Ar Source: http://www.webelements.com/periodicity/abundance_crust/ X II Fe N Ar Ar oxidized ligand, FeII Chirik and Wieghardt have proposed that redox active ligands may be able to confer nobility on base metals by playing the role of electron sinks. reduced ligand, FeII X X F (10 mol%) Science, 2010, 327, 794. X X 23 °C Bis(aryliminopyridine) Pincer Ligand: Conversion > 90% for X = CH2, NHBn, NHtBu, C(CO2Et)2 after 5h (< 30 min for amine compounds) Chirik, J. Am. Chem. Soc., 2006, 128, 13340. F' (2.5 mol%) Me iPr II N Fe Br iPr 0.5% Na(Hg) or 2 NaC10H8 Me N N iPr Br iPr Me iPr N2 Me N II N Fe N2 iPr + N iPr 23 °C, 16 h 95% F' (2.5 mol%) Me N2 + 23 °C, 16 h 95% iPr Via: This ligand framework can store two reducing equivalents in an iron(II) complex. It has been used for several reactions: * Formal [2 + 2] cycloadditions R=H reductive elimination (For F' structure, see next slide) N N Fe Me N * Hydrogenation and hydrosilylation * Oxidative additions into C–C bonds Me Me Me * 1,6-enyne reactions Me Me Ar R = Me β-elimination R then reductive elimination Me Me Chirik, J. Am. Chem. Soc. 2011, 133, 8858. 8 Redox-Active Ligands Q. Michaudel 1,6 enyne reactions: F (5 mol%) E E 23 °C, H2 (4 atm) PhH, 23 °C Me E = NTs, R = H, 79% (1h) R = Me, 79% (3h) E = O, R = H, 95% (6h) R = Me, 62% (3h) E = C(EtCO2)2, R = H, 74% (3h) (TMSO)2MeSiH F' (0.004 mol%) 5 Me Me R II N Fe E 5 Me Ph Me 23 °C, 30 min, > 98% Si N F Ar Fe N N N Me Fe R N Ph N F': R = Me F": R = Et R iPr R Me N N Ar Me N2 N2 iPr N N OTMS OTMS (TMSO)2MeSiH F' (0.004 mol%) Me N Me 23 °C, neat 15 min, > 98% N OTMS OTMS Si N Chirik, J. Am. Chem. Soc., 2009, 131, 8772. iPr N Anti Markovnikov Hydrosilylation: R R Baran Lab GM 2013-10-19 F iPr O E (TMSO)2MeSiH F' (0.26 mol%) Me O 8.9 60 °C, 60 min, > 98% Me OTMS OTMS Si O O Me 8.9 Chirik, Science, 2012, 335, 567. Me Ar N II N Fe H H Me Me N R Ar Me N N II Fe Enantioselective Hydrogenation: Me N R Ar Ar N N H2 E iPr N Cy R1 Me F iPr – 2 N2 22 °C, H2 (4 atm) PhH, 24 h R2 R1 R2 R1 = Ar, R2 = Alk Up to > 98% yield, 96% ee G Hydrogenation and hydrosilylation: Me G (5 mol%) Co Me E Me Chirik, J. Am. Chem. Soc., 2012, 134, 4561. Me [Fe] This catalyst enables the hydrogenation of olefins in the presence of unprotected amines, various carbonyls, ethers and fluorinated hydrocarbons. Reduction of Azides: N3 [Fe] F (10 mol%) [Fe] Me H [Fe] H Chirik, J. Am. Chem. Soc., 2004, 126, 13794; Organometallics, 2008, 27, 1470. NH2 23–65 °C, H2 (4 atm) Me H2 R R: R 2,6-iPr 2 > 2,5-tBu 2 > 2,6-Et2 >>> 2,4,6-Me3 Chirik, J. Am. Chem. Soc., 2006, 128, 5302. Me 9 Redox-Active Ligands Q. Michaudel Oxidative additions into C–C bonds: Me iPr II N Fe Me iPr N 23 °C, 2 h N2 N2 Negishi-type cross coupling: Me N Ar N III Fe Cl– EtCl Me N N Ar tBu O iPr iPr Baran Lab GM 2013-10-19 tBu III tBu O Co tBu O N Ph Ph N III tBu Co O N Ph tBu In this case, both ligand and metal give one electron. This is another way for this bis(imino)pyridine frame to adjust to the electronic requirements of a metal complex and a specific redox process. Me tBu Ph N tBu Chirik, J. Am. Chem. Soc., 2012, 134, 17125. J. Am. Chem. Soc., 2010, 132, 14358. C–C Bond Formation: This paper set a key precedent for the field of redox-active ligands, because it showcases a reductive elimination from a ZrIV d0 complex!! 2 Ph Ph tBuN Zr Ph Ph IV O O tBuN tBu [Cp2Fe]PF6 (2 equiv.) tBu tBu tBu tBuN Heyduk, J. Am. Chem. Soc., 2006, 128, 8410. Me Me tBuN tBu tBu Zr Reaction also works with hexylzinc bromide in similar yields. O tBu tBu THF O reduced ligand, ReV Ph3P O O O O V Re O O O O O O O tBuN tBu Zr IV O tBuN 2 Ph3P O oxidized ligand, ReV H tBu O tBu O VII O O tBu O O O O tBu tBu O V Re H IV O tBuN J. Am. Chem. Soc., 2010, 132, 3879. Dioxygen Fixation: Ph Ph (yield: 74%) "Authentic carbon-carbon bond-forming reductive elimination reactions are uncommon outside of the platinum group triad." PhZnBr Miscellaneous Reactions: O tBuN tBu tBu Zr IV PhEt + [ZnBr]+ (yield: 10-15%) The dimethyl equivalent yields 23% of a mixture of methane (79%) and ethane (21%), so about 5% yield for the Me–Me coupling. reduced ligand, ReVII O Re O O O O Re V O O O O V Re O O O O O O oxidized ligand, ReV The ligand facilitates spin-crossover in the formally “spin-forbidden” reaction between triplet oxygen and the closed-shell (singlet) d2 rhenium(V). 10 Redox-Active Ligands Q. Michaudel Nitrene Transfer: MeO The elegant work of Milstein and Tanaka on base assisted redox-active ligand catalysis for alcohol oxidation is also notable: iPr N tBu N C N IV 2 Cl NH2 Zr CNtBu N R Baran Lab GM 2013-10-19 N RN3 III Ru N iPr N NH – H+ N O N III Ru + H+ O N N N N O II Ru N O N O O MeO MeO N2 CNtBu iPr tBu MeO iPr N N IV tBu tBu tBu tBu tBu 1/2 MeOH N Cl N C N Zr N N R iPr MeO Cl IV tBu Zr Tanaka, J. Am. Chem. Soc., 2003, 125, 6729. NR CNtBu R1NH2 + R2CH2OH (1 equiv.) (1 equiv.) N iPr MeO MeO J (0.1 mol%) R1NHCOR2 + 2H2 PhMe, reflux up to 96% R1, R2 = Alk, Ar N iPr R = Ad, tBu N N Cl NR J NtBu Co tBu tBu Me "The redox activity of iminopyridine ligands may play a role in effecting efficient catalysis". N Me I Ar Four-Electron Oxidative Formation of Aryl Diazenes Using a Tantalum Redox-Active Ligand Complex: Heyduk, Angew. Chem., Int. Ed., 2008, 47, 4715. Ar Iron Diazoalkane Chemistry: N–N Bond Hydrogenation and Intramolecular C–H Activation: Chirik, J. Am. Chem. Soc., 2007, 129, 7212. N Fe Cl2 Further Reading: Catalytic Reactivity of a Zirconium(IV) Redox-Active Ligand Complex with 1,2-Diphenylhydrazine: Heyduk, J. Am. Chem. Soc., 2008, 130, 2728. Ritter, J. Am. Chem. Soc., 2009, 131, 12915. Me O 2 “Oxidative Addition” to a Zirconium(IV) Redox-Active Ligand Complex: Heyduk, Inorg. Chem., 2005, 44, 5559. Coupling of tBuNC with AdN3 reacted to completion in 2 h at 55 °C with 10 mol% of catalyst. I (4 mol%) Mg (10 mol%) Me HBPin (1.2 equiv.) Me Et2O, 23 °C, BPin 3 h, 80 °C H N O Milstein, Science, 2009, 324, 74. Heyduk, Chem. Sci., 2011, 2, 166. MeO PtBu Ru Et2N iPr Hydroboration of 1,3 Diene: II N N Zr NH2 Ru N IV 1/2 HCOH Ar = 3,5-dimethylphenyl Reaction of a Redox-Active Ligand Complex of Nickel with Dioxygen Probes Ligand-Radical Character: J. Am. Chem. Soc., 2009, 131, 15582. 11