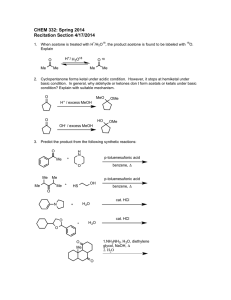
CHEM 332 – Spring 2016 Name: Problem Set 4
advertisement
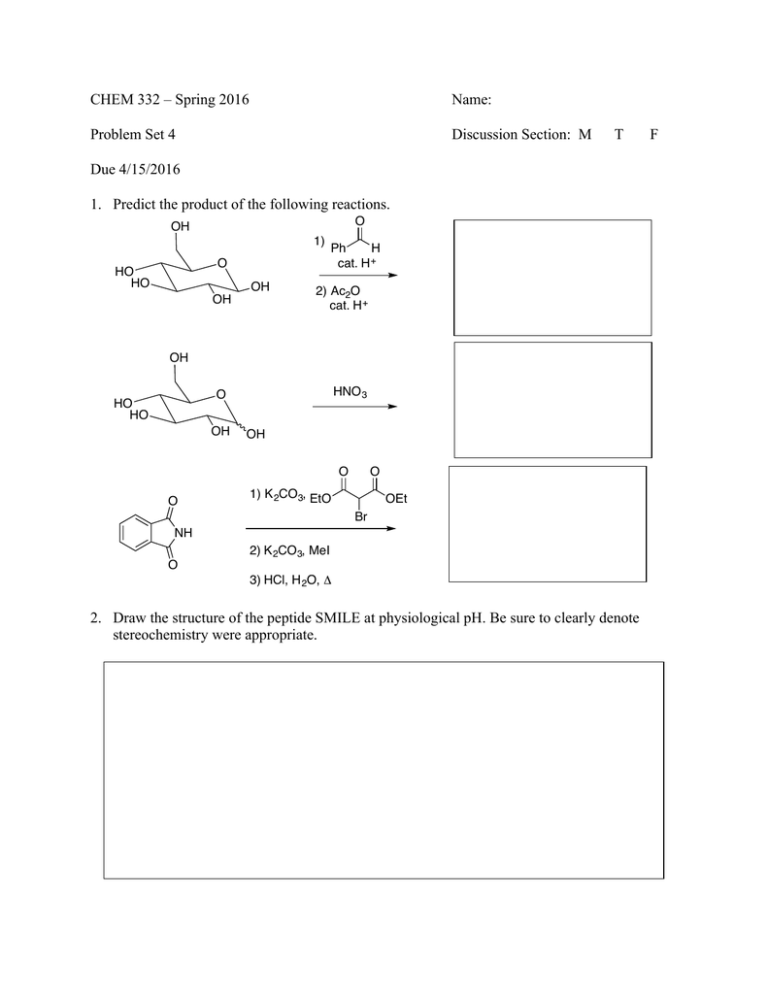
CHEM 332 – Spring 2016 Name: Problem Set 4 Discussion Section: M T Due 4/15/2016 1. Predict the product of the following reactions. O OH 1) O HO HO OH OH Ph H cat. H + 2) Ac2O cat. H + OH HNO 3 O HO HO OH OH O O O 1) K 2CO 3, EtO OEt Br NH 2) K 2CO 3, MeI O 3) HCl, H 2O, Δ 2. Draw the structure of the peptide SMILE at physiological pH. Be sure to clearly denote stereochemistry were appropriate. F 3. Provide a reasonable arrow-pushing mechanism for the following reactions. a) OH OH HO HO HO CH 3CH2OH, cat. H + O HO HO HO O OH OEt b) S OH cat. HCl H HO HO HO O OH SH OH S OH H H OH H OH CH2OH 4. Provide a reasonable arrow-pushing mechanism for the following reaction and explain where each oxygen came from in the final product. O O OH H 3C O HO HO OH OH O O cat. H + CH 3 O CH 3 O O H 3C O H 3C O O O O O O CH 3 CH 3 5. Propose a synthesis of isoleucine from butanal. You may use any inorganic reagents and any organic reagent with less than 2 carbons. O O H H 2N OH 6. Base pair mismatch in DNA is often detrimental and can have severe consequences. In the case of a mismatch, it is possible to form a non-canonical base pair in order to maximize the number of hydrogen-bonding interactions and minimize steric repulsion between the bases. Using your understanding of organic chemistry, propose plausible structure for the bonding interaction between an A-G mismatch. N H 2N H H N N Adenine N H N N N N Guanine N O No hydrogen bonds & steric clash therefore this interaction is bad!