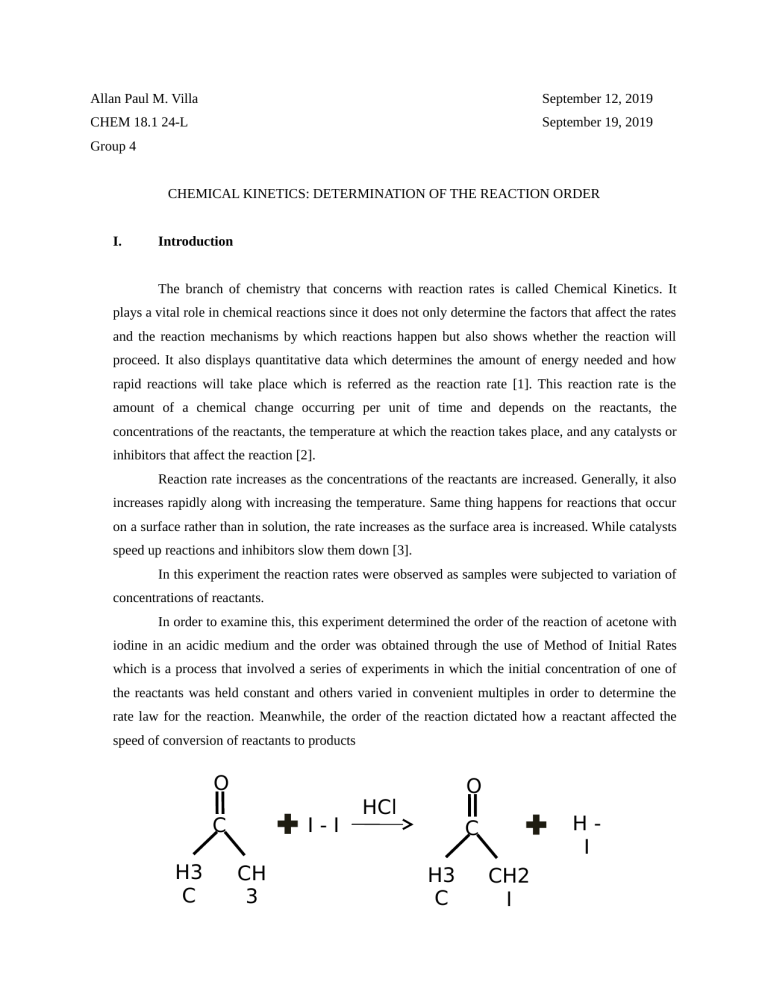
Allan Paul M. Villa September 12, 2019 CHEM 18.1 24-L September 19, 2019 Group 4 CHEMICAL KINETICS: DETERMINATION OF THE REACTION ORDER I. Introduction The branch of chemistry that concerns with reaction rates is called Chemical Kinetics. It plays a vital role in chemical reactions since it does not only determine the factors that affect the rates and the reaction mechanisms by which reactions happen but also shows whether the reaction will proceed. It also displays quantitative data which determines the amount of energy needed and how rapid reactions will take place which is referred as the reaction rate [1]. This reaction rate is the amount of a chemical change occurring per unit of time and depends on the reactants, the concentrations of the reactants, the temperature at which the reaction takes place, and any catalysts or inhibitors that affect the reaction [2]. Reaction rate increases as the concentrations of the reactants are increased. Generally, it also increases rapidly along with increasing the temperature. Same thing happens for reactions that occur on a surface rather than in solution, the rate increases as the surface area is increased. While catalysts speed up reactions and inhibitors slow them down [3]. In this experiment the reaction rates were observed as samples were subjected to variation of concentrations of reactants. In order to examine this, this experiment determined the order of the reaction of acetone with iodine in an acidic medium and the order was obtained through the use of Method of Initial Rates which is a process that involved a series of experiments in which the initial concentration of one of the reactants was held constant and others varied in convenient multiples in order to determine the rate law for the reaction. Meanwhile, the order of the reaction dictated how a reactant affected the speed of conversion of reactants to products O C H3 C I-I CH 3 O HCl HI C H3 C CH2 I In determining the rate law, numerous steps were done. The experiment was performed in a systematic manner and information were gathered and presented in tabular forms. The concentrations of the reactants in reaction mixtures were calculated first followed by the determination of the reaction rates. These reaction rates were summarized into rate laws which described the relation between the velocity of a reaction and the concentration of chemical reactants. For the general reaction aA+bB→C with no intermediate steps in its reaction mechanism, meaning that it is an elementary reaction, the rate law is given by: Rate = k [A]x [B]y [C]z In this equation [A], [B] and [C] expressed the concentrations of acetone, iodine and hydrochloric acid, respectively, in units of moles per liter. The exponents x, y and z are the individual orders, and k is the rate constant [4]. Using the same formula, the ratio of rate law was determined to calculate the values of x, y and z or the orders of the reaction with respect to the reagents which are considered to be the foundation of the rate law expression [5]. After obtaining the values, these were plugged in all the trials to get the rate constant. These rates constants were then averaged and the calculated value together with the calculated orders was used in the writing of the final rate law of the reaction. Similar study (Iodination reaction) began in 19th century when it was noticed that the color of iodine slowly fades when the solution also contains acetone. Since then, reactions involving a reactant containing a central carbonyl group have become some of the popular organic chemical reactions. The iodination of acetone is also catalyzed by hydrogen ions. The effects of varying the concentrations of acetone, iodine and hydrogen ions have been studied earlier and it has been found that the reaction is zero order with respect to iodine. [6] I. Materials A. Reagents 4 M Acetone 0.0012 M I2 1 M HCl starch solution B. Apparatus and Equipment Test Tube Rack 5 Test Tubes 5 125-mL Erlenmeyer Flasks 10-mL Graduated Cylinder Rectangular Container II. Procedure To show the dependence of the rate of reaction upon the concentration of reactants, certain amounts of HCl, I2, starch and water with a final volume of 25mL (as summarized in Table 1) were placed in the Erlenmeyer flasks. Each flask was labelled trial 1 to 5 in order to avoid confusion and errors as the experiment wore on. Subsequently, needed volume of acetone were put in the test tubes and were labelled trial 1 to 5 too. After this, the flasks and test tubes were soaked in the water bath and allowed the temperature to equilibrate for 2 minutes. Trial 1 2 3 4 5 4 M Acetone 2.50 5.00 2.50 2.50 3.75 0.0012 M I2 2.50 2.50 2.50 5.00 3.75 Volume, mL 1 M HCl 2.50 2.50 5.00 2.50 3.75 Starch Solution 2.50 2.50 2.50 2.50 1.25 H 2O 15 12.50 12.50 12.50 12.50 Table 1. Volume of Reagents After stabilizing the temperature of the samples, the test tubes and flasks were removed in the water bath and the content of the test tubes were poured into the respective Erlenmeyer flasks. The timer was then started as soon as the acetone was transferred to the flasks. The mixtures were swirled in a constant direction and in the same manner until the dark blue color no longer persisted. To ensure this, the mixture was observed in a white background. The time that the color disappeared in each flask and became clear was recorded on Table 2.1. Finally the group cleaned up the equipment and solutions used in the experiment. The solutions were disposed of in the waste container labelled E501. Then the glasswares were cleaned in the sinks. III. Data/Observations After performing the experiment, the following sets of information were gathered. Run 1 2 3 4 5 [I2] in mixture, M 1.2x10-4 1.2x10-4 1.2x10-4 2.4x10-4 1.8x10-4 Time started, s 0 0 0 0 0 Time finished, s 327 305 315 1048 180.05 Time elapsed, s 327 305 315 1048 180.05 -d[I2]/dt, M/s 3.7x10-7 3.9x10-7 3.8x10-7 2.3x10-7 1.0x10-6 Table 2.1. Determination of the Various Reaction Rates Table 2.1 shows the initial concentration of Iodine in each trial calculated based upon the stock concentration and the volumes listed in Table 1 under the formula C 1V1=C2V2. Where C1 is the Molarity of the given substance, V1 is the volume used of that substance, C 2 is the total Molarity used and V2 is the total volume used. The reaction time were recorded on this table in seconds and the reaction rates were calculated as the change in concentration of iodine over reaction time or through the equation, Rate of Reaction = Run 1 2 3 4 5 [C3H6O] 0.40 0.80 0.40 0.40 0.60 −∆[ I ]2 . ∆t Concentration in the Mixture, M [I2] 1.2x10-4 1.2x10-4 1.2x10-4 2.4x10-4 1.8x10-4 [HCl] 0.10 0.10 0.20 0.10 0.15 Reaction Rate, M/s 3.7x10-7 3.9x10-7 3.8x10-7 2.3x10-7 1.0x10-6 Table 2.2. Determination of the Reaction Order While Table 2.2 shows the initial concentration of the other reagents (acetone and HCl) per run calculated based upon the values in Table 1 under the same formula C 1V1=C2V2 used in finding the initial concentration of iodine. The reaction rate can also be seen on this table. Reactant Reaction Order with Respect to Reactant I2 C3H6O HCl -1 0 0 Effect of Doubling Concentration to the reaction time (increase/decrease/no effect) Increase Decrease Decrease Lastly, Table 3 shows the reaction orders with respect to the reactants which were obtained by finding the values of x, y and z through utilizing the rate law equation Rate = k [A]x [B]y [C]z. In order to get these values, ratio of the rate laws for each trial where in specific reactants were set constant was used. To find the order of Iodine (y), Trials 1 and 4 were treated. Following the formula r1 r4 = k [ A]1x [B] 1y [C ]1z , where r1 and r4 is the reaction rate on runs 1 and 4, respectively; [A], k [ A ]4x [B] 4y [C ]z4 [B] and [C] is the molarity of acetone, iodine and HCl, respectively the obtained value is -0.69, rounding this off gives us a value of -1. On the other hand, using the same treatment, the calculated order of acetone (x) using trials 1 and 2 is 0.076 or 0 while the order of HCl (z) using trials 1 and 3 is 0.038 or 0 when rounded in the nearest whole number. Also, the effect of doubling the concentration of either of the reactants to the reaction time is transparent on this table. In trial 2, the amount of acetone was doubled (see table 1) and it can be seen in table 2.1 that the reaction time decreased. The same manner happened to HCl when its volume was doubled in trial 4 (see table 1). Meanwhile, when the volume of iodine was doubled, there was an increase in reaction time. After x, y and z had been determined, the rate constant or k was calculated. This was done by using each reaction and determining the individual k, then averaging all of them to determine the overall rate constant. Using the equation Rate = k [A]x [B]y [C]z, k is determined from a simple algebraic equation of dividing the rate by the concentration of A, B and C all taken to its order. Simply written, it is k = R . Upon plugging the values, this gave a rate constant of [ A ] [ B]y [C] z x 8.9x10-10 M-1 sec-1 on runs 1 to 4 and 3.0x10-9 M-1 sec-1 on run 5. Hence, the computed average rate constant is 1.3x10-9 M-1 sec-1. IV. Discussion Based on the results, when we qualitatively compare the standard run (run 1) to the doubled acetone run (run 2) the rate of the reaction barely changed; therefore we can infer that the reaction is zero order with respect to acetone. Same thing can be drawn when we compare the standard run to the doubled acid run (run 3). There was no change in reaction rate at all, thus, the reaction is zero order with respect to HCl. Similar conclusions can be inferred using the calculated value (0.08 and 0.04 or roughly 0) of each ratio of the runs. While, upon comparing the standard run and the doubled iodine run (run 4) the reaction rate decreased and quantitatively it gave us -0.68 or -1 value. With these values and the aforementioned average rate constant, we can now write the final rate law as: Rate = 1.3x10-9 M-1 sec-1 [C3H6O]0[I2]-1[HCl]0. V. Conclusion A series of steps is required to determine the rate law of a chemical reaction. By varying the concentrations of reactants in the iodination of acetone it was determined that the reaction is -1 order with respect to the iodine concentration and zero order with respect to both acetone and hydrochloric acid concentration. Through the reaction rate we can also determine which reaction is the fastest and slowest. In this experiment the 5 th run is the fastest and the 4 th run is the slowest in accordance with varying concentrations of the reactants. Through analyzing the calculated reaction order the reaction rates were independent of that of the concentrations of acetone and hydrochloric acid or the rates did not changed even if the concentrations were doubled. Consequently, the negative 1 order value with respect to iodine indicates that the iodine concentration is inversely proportional to the rate of the reaction. Unfortunately, these obtained results did not matched up with the expected ones. The reaction should have been 1st order with respect to acetone and HCl which means that these two should have been directly proportional to the increase of their concentrations. Meaning, as their concentrations were doubled, the reaction rate should be doubled too. On the other hand the reaction should have been zero order with respect to iodine. There should have been no relative change in the reaction rate when the iodine concentration was doubled [6]. With this, we can say that there could be errors committed while performing the experiment. The experiment done might have been subjected to different factors. There could have been wrong measurements of the reactants and/or there was a relative difference while swirling the mixture. Impurities in the reactants could also be one factor that affected the results as well as the temperature. The allotted 2 minutes could have not been enough to well-equilibrate the samples and cancel this variable on the determination of the reaction rates. VI. Literature Cited/Bibliography [1] Retrieved June 6, 2019 (September 17, 2019) from the LibreTexts: http://www.libretexts.org [2] Retrieved February 2, 2010 (September 17,2019): cms.montgomerycollege.edu [3] HILL JW, PETRUCCI RH, MC CREARY TW, PERRY SS. 2010. Rates and Mechanisms of Chemical Reactions. In: General Chemistry Fourth Edition. USA: Pearson Education Ed. Chapter 13. [4] Retrieved April 1, 2014 (September 17, 2019) from the Odinity: www.odinity.com [5] Retrieved February 2, 2010 (September 17, 2019) from the MNState: web.mnstate.edu [6] SHUKLA ST, KULKARNI S. 2015. Kinetics of iodination of acetone, catalyzed by HCl and H2SO4. India. 7(8):226-229 Sample Calculations Calculating the initial concentration of I2 in the mixture Runs 1, 2 & 3 I ¿ volume of I 2 ¿ stock ¿ [I2]initial = volume of solution¿ = total ¿ ¿ ¿ ¿ (0.0012 M )(2.50 mL) 25 mL = 1.2x10-4M (0.0012 M )(5.00 mL) 25 mL = 2.4x10-4M (0.0012 M )(3.75 mL) 25 mL = 1.8x10-4M Run 4 I ¿ volume of I 2 ¿ stock ¿ [I2]initial = volume of solution¿ = total ¿ ¿ ¿ ¿ Run 5 I ¿ volume of I 2 ¿ stock ¿ [I2]initial = volume of solution¿ = total ¿ ¿ ¿ ¿ Calculating the initial concentration of C3H6O in the mixture Runs 1, 3 & 4 volume of acetone ¿stock ¿ volume of solution ¿total [acetone]initial = = ¿ acetone¿ stock ¿ ¿ ¿ Run 2 (4 M )(2.50 mL) 25 mL = 0.40M volume of acetone ¿stock ¿ volume of solution ¿total [acetone]initial = = ¿ acetone¿ stock ¿ ¿ ¿ (4 M )(5.00 mL) 25 mL = 0.80M (4 M )(3.75 mL) 25 mL = 0.60M Run 5 volu me of acetone ¿stock ¿ volume of solution¿total [acetone]initial = ¿ acetone¿ stock ¿ ¿ ¿ = Calculating the initial concentration of HCl in the mixture Runs 1, 2 & 4 volume of HCl ¿ stock ¿ volume of solution ¿ total [HCl]initial = = ¿ HCl ¿stock ¿ ¿ ¿ (1 M )(2.50 mL) 25 mL = 0.10M (1 M )(5.00 m L) 25 mL = 0.20M (1 M )(3.75 mL) 25 mL = 0.15M Run 3 volume of HCl ¿ stock ¿ volume of solution ¿ total [HCl]initial = = ¿ HCl ¿stock ¿ ¿ ¿ Run 5 volume of HCl ¿ stock ¿ volume of solution ¿ total [HCl]initial = = ¿ HCl ¿stock ¿ ¿ ¿ Calculating the rate of reaction Run 1 Rate of Reaction = – I [¿¿ 2] ∆ ∆t ¿ = – I 2 ¿initial ¿ I 2 ¿ final−¿ ¿ ¿ = 0 M −1.2 x 1 0−4 M 327 sec = – I 2 ¿initial ¿ I 2 ¿ final−¿ ¿ ¿ = 0 M −1.2 x 1 0 305 sec = – I 2 ¿initial ¿ I 2 ¿ final−¿ ¿ ¿ = 0 M −1.2 x 1 0 315 sec = – I 2 ¿initial ¿ I 2 ¿ final−¿ ¿ ¿ = 0 M −2.4 x 1 0− 4 M 1048 sec = – I 2 ¿initial ¿ I 2 ¿ final −¿ ¿ ¿ = 0 M −1.8 x 10 M 180.05 sec = 3.7x10-7 M sec Run 2 Rate of Reaction = – I [¿¿ 2] ∆ ∆t ¿ −4 M = 3.9x10-7 M sec Run 3 Rate of Reaction = – I [¿¿ 2] ∆ ∆t ¿ −4 M = 3.8x10-7 M sec Run 4 Rate of Reaction = – I [¿¿ 2] ∆ ∆t ¿ = 2.3x10-7 M sec Run 5 Rate of Reaction = – M sec I [¿¿ 2] ∆ ∆t ¿ −4 = 1.0x10-6 Comparing run 1 (standard run) and run 4 (doubled iodine) x r1 r4 y = M sec M 2.3 x 10−7 sec = = M sec M 3.9 x 10−7 sec = = M sec M 3.8 x 10−7 sec = −7 3.7 x 10 z k [ A]1 [B] 1 [C ]1 x y z k [ A ]4 [B] 4 [C ]4 = k [0.40 M ]1x [1.2 x 1 0−4 M ]1y [0.10 M ]1z k [0.40 M ]x4 [2.4 x 1 0−4 M ]4y [0.10 M ]4z M sec ln M 2.3 x 1 0−7 sec −4 y [1.2 x 1 0 M ] ln [2.4 x 1 0−4 M ]❑y 3.7 x 1 0 −7 = y = – 0.69 Comparing run 1 (standard run) and run 2 (doubled acetone) r1 r2 k [ A ]1x [B]1y [C ]z1 k [ A ]2x [B]2y [C ]z2 = x −4 y 3.7 x 10−7 z k [0.40 M ]1 [1.2 x 10 M ]1 [0.10 M ]1 x −4 y z k [0.80 M ]2 [1.2 x 1 0 M ]2 [0.10 M ]2 M sec ln M 3.9 x 1 0−7 sec x [ 0.40 M ] ln x [0.80 M ]❑ 3.7 x 1 0 −7 = x = 0.076 Comparing run 1 (standard run) and run 3 (doubled HCL) r1 r3 = x 1 x 3 y 1 y 3 z 1 z 3 k [ A ] [B] [ C ] k [ A ] [B] [ C ] k [0.40 M ]x1 [1.2 x 10−4 M ]1y [0.10 M ]1z k [0.40 M ]x3 [1.2 x 1 0−4 M ]3y [0.20 M ]3z 3.7 x 10−7 M sec ln M 3.8 x 1 0−7 sec [0.10 M ] z ln [0.20 M ]❑z 3.7 x 1 0−7 = z = 0.038 Calculating the rate constant (k) Run 1 Rate = k [C3H6O]0[I2]-1[HCl]0 Rate = k (0.40)0(1.2x10-4)-1(0.10)0 M 0.40 ¿ ¿ 0.10 M ¿ 0 k = 1.2 x 10−4 M ¿−1 ¿ = ¿ −7 M 3.7 x 10 sec ¿ 8.9x10-10 M-1 sec-1 Run 2 Rate = k [C3H6O]0[I2]-1[HCl]0 Rate = k (0.40)0(1.2x10-4)-1(0.10)0 M 0.80 ¿ ¿ 0.10 M ¿ 0 k = 1.2 x 10−4 M ¿−1 ¿ = ¿ −7 M 3.9 x 1 0 sec ¿ 8.9x10-10 M-1 sec-1 Run 3 Rate = k [C3H6O]0[I2]-1[HCl]0 Rate = k (0.40)0(1.2x10-4)-1(0.10)0 M 0.40 ¿ ¿ 0 0.20 M ¿ k = 1.2 x 10−4 M ¿−1 ¿ = ¿ M 3.8 x 10−7 sec ¿ 8.9x10-10 M-1 sec-1 Run 4 Rate = k [C3H6O]0[I2]-1[HCl]0 Rate = k (0.40)0(1.2x10-4)-1(0.10)0 M 0.40¿ ¿ 0.10 M ¿0 k = 2.4 x 1 0−4 M ¿−1 ¿ = ¿ −7 M 2.3 x 1 0 sec ¿ 8.9x10-10 M-1 sec-1 Run 5 Rate = k [C3H6O]0[I2]-1[HCl]0 Rate = k (0.40)0(1.2x10-4)-1(0.10)0 M 0.60 ¿ ¿ 0.15 M ¿ 0 k = 1.8 x 1 0−4 M ¿−1 ¿ = ¿ −6 M 1.0 x 10 sec ¿ 3.0x10-9 M-1 sec-1 Calculating the average rate constant kave = ∑k n −10 kave = 4( 8.9 x 1 0 −1 −1 −9 −1 −1 M se c )+3.0 x 10 M se c 5 kave = 1.3x10-9 M-1 sec-1