Experiment 9: Synthesis of Benzoic Acid

Experiment 9: Synthesis of Benzoic Acid via Carbonylation of a Grignard Reagent
Organometallic compounds such as Grignard reagents are versatile intermediates in the synthesis of alcohols, carboxylic acids, alkanes and ketones.
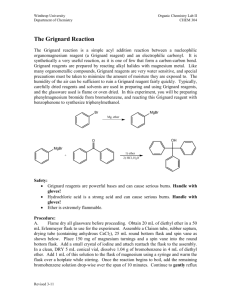
The synthesis of a Grignard reagent requires the reaction of an alkyl or aryl halide with magnesium metal. In this experiment, you will be reacting bromobenzene with magnesium to yield phenylmagnesium bromide. Often
Grignard reagents are prepared under inert atmospheres such as nitrogen to prevent its reaction with oxygen and water. The preparation that you will be doing does not use rigorously anhydrous conditions but the phenylmagnesium bromide will be nonetheless protected from oxygen by a blanket of refluxing ether.
Br MgBr
Mg dry ether bromobenzene phenylmagnesium bromide
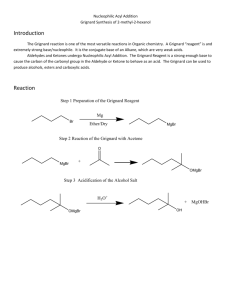
Reaction of the Grignard reagent, phenylmagnesium bromide with carbon dioxide gives benzoic acid after acid hydrolysis.
O OMgBr
MgBr C
CO
2 dry ether phenylmagnesium bromide addition complex
O
C
OMgBr addition complex
O
C
OH
H
2
O
HCl
benzoic acid
Procedure:
A. Preparation of the Grignard Reagent
Fit a dry (Note 1) 25 mL round bottom flask with a condenser. Remove the condenser and add to the flask 5 mL of anhydrous ether, 0.130 grams (5.3 mmoles) of magnesium metal turnings, 0.5 mL (0.75 grams; 4.8 mmoles) of bromobenzene, and a magnetic stir bar. The start of the reaction is indicated by a slight cloudiness of the solution and then the boiling of the ether. After the vigorous reaction subsides, gently boil and stir the reaction mixture for another
15 minutes.
Notes
1. All glassware and materials must be dry. Clean glassware that has been stored in the lab drawer is sufficiently dry. Freshly washed glassware should be rinsed repeatedly with acetone, air dried to evaporate the acetone, and heat dried in an oven for a few minutes prior to using.
B. Grignard Reaction
Remove the heating bath from the Grignard reagent mixture. Place approximately 1 to 2 grams of crushed solid carbon dioxide (Dry Ice) into a 50mL beaker (Note 1). Carefully pour the ether solution of phenylmagnesium bromide onto the crushed Dry Ice. Stir the mixture until all of the carbon dioxide has either reacted or evaporated leaving a pasty or gelatinous product residue.
Add 10 mL of water and carefully add 3 mL of 6N hydrochloric acid and stir the mixture thoroughly. Transfer the aqueous mixture to a separatory funnel and extract it twice with 10-mL portions of ether. Combine the ether extracts and separate and set aside the aqueous phase. (Note 3.) Place the combined ether layers back in the separatory funnel and extract them them with two 10-mL portions of 5 percent sodium hydroxide solution. If there are any solids present in the basic extract, filter the extract to remove the solids. The extract is acidified to pH 3 (Note 2) with 6N hydrochloric acid. After cooling, the precipitated solid is isolated by suction filtration. The collected solid is recrystallized from water to yield benzoic acid as fine, light, colorless needles, mp. 122-123°C. Determine the
% yield and melting point of the recrystallized product.
Notes
1. The crushed dry ice will be available in an ice bucket in the hood. Try to avoid using Dry Ice from the outer surface of the crushed material which contains frost from moisture condensed on the surface. Dig just below the surface to avoid frost. It is not necessary to weigh out the dry ice. Just estimate the amount for 1-2 grams, transfer it quickly to a beaker and use it right away.
2. Use pH indicator paper to check the pH.
3. This aqueous layer will be discarded at the end of the experiment, but it is a good idea to save all layers until the end. That way if there is some kind of a mix-up in the layers, you will not accidentally throw out the layer containing your product.