Key
advertisement

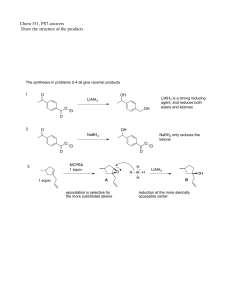
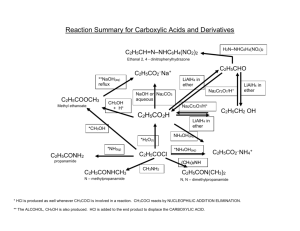
Chem 331, PS #8 due Mon, Nov 11 , 2002 use extra paper (use a staple or paper clip) Key Name 1. Match each compound with its IR spectrum. OH For A, steric hinderance prevents H-bonding. Thus, the OH stretch is sharp. OH A B B 3300 cm–1 A 3620 cm–1 SHARP! The syntheses in problems 2-4 all give racemic products 2) O OH LiAlH4 is a strong reducing agent, and reduces both esters and ketones LiAlH4 O OH Et O 3) HO O NaBH 4 O NaBH 4 only reduces the ketone O Et MCPBA 1 equiv. 4) 1 equiv. Et O O O A epoxidation is selective for the more substituted alkene H – H Al H H LiAlH4 OH B reduction at the more sterically accessible center 5) Propose a multistep synthesis O Retrosynthesis: this is your scratch work! We find that there is more than one possibility This type of arrow Williamson ether synthesis a refers to a "backwards" transformation Br OH O a O b BrMg PBr3 b Br Williamson ether synthesis OH Points about the above retrosynthesis • Path a is better than b because it is shorter, and because it uses an allylic bromide rather than a secondary bromide (we expect a better yield with the more reactive allylic bromide in an SN2 reaction). • We worked our way back to a 5 carbon intermediate epoxide, because we knew that this could be constructed from our starting alkene. The forward synthesis: ( this is your formal answer!) O O Cl OH O BrMg OH ether racemic 1) NaH (MCPBA) 2) note: NaH is being used as a base to deprotonate the alcohol OH NaH Br O O– Na+