Prelab for “Preparation and Carbonation of a Grignard Reagent
advertisement

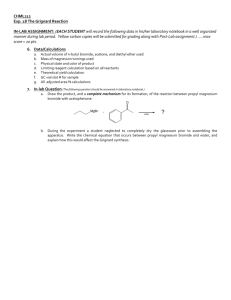
Prelab for “Preparation and Carbonation of a Grignard Reagent: Synthesis of Benzoic Acid” Name: Section: 1. Write a chemical reaction showing the product expected from the reaction between phenylmagnesium bromide and dry ice. Then write another chemical reaction showing the product from phenylmagnesium bromide and acetone. Comment on the similarity of these two reactions. 2. Benzoic acid does not dissolve in cold water to any great extent. However, when the solid is added to 5% cold aqueous NaOH solution, it completely dissolves. Explain this fact with the help of chemical equations. 3. Look up the 1H chemical shift for the COOH proton. Explain why it is so deshielded. 4. Explain why the IR frequency for the OH in benzoic acid is lowered to about 3000 cm-1 from 3300 cm-1 observed for a normal alcohol.