Grignard Reaction: Benzoic Acid Synthesis Lab Report
advertisement

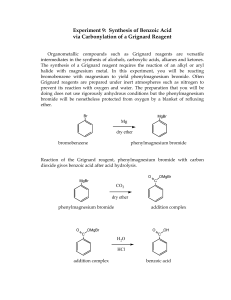
The Grignard Reaction Organic Chemistry 2580 Laboratory, Section 01 Experiment 18 10/31/18 I. Abstract The Grignard reaction is considered to be one of the most important reactions in organic chemistry with great importance and elegance. This reaction results in high yields and can be performed using 1, 2, 3, and aromatic halides. In this laboratory experiment the goal was to synthesize benzoic acid by preparing a Grignard reagent from bromobenzene. Under anhydrous conditions magnesium metal was added to bromobenzene to form phenylmagnesium bromide which was then reacted with CO 2 and to give an intermediate benzoate ion which was then in turn reacted with HCl to give the product, benzoic acid. The % yield and melting range were determined to be 62% and 114-115C respectively. II. Introduction The alkyl group of the prepared Grignard reagent acts as a very strong base and will thus react with acids to generate an alkane. However, this also means that any compound that contains H+ will donate that proton and in turn will destroy the Grignard reagent. This is why the glassware were all baked in the oven and dried subsequently over a flame. Iodine crystals are used to activate the magnesium metal. When phenylmagnesium bromide is reacted with CO2 it’s CO2 that acts as the electrophile to yield the intermediate benzoate ion. HCl is then added in order to neutralize the benzoate ion protonating it and converting it to benzoic acid. H2O serves as a solvent for the magnesium salts. Extraction steps were performed based on polarity and solubility differences in order to remove impurities or by-products such as biphenyl which is formed by phenylmagnesium bromide reacting with bromobenzene. Below is the mechanism for the reduction of diphenylmethanol from benzophenone: III. Results Name Benzoic acid Molar Mass 122.12 g/mol Yield 4.61 g Structural formula Melting range 114-115C Theoretical yield 7.44 % Yield 62% Appearance IV. White solid powder Conclusion In this laboratory experiment the goal was to synthesize benzoic acid by preparing a Grignard reagent from bromobenzene. Under anhydrous conditions magnesium metal was added to bromobenzene to form phenylmagnesium bromide which was then reacted with CO2 and to give an intermediate benzoate ion which was then in turn reacted with HCl to give the product, benzoic acid. The % yield and melting range were determined to be 62% and 114-115C respectively. The % yield was higher than that reported in the lab manual, which was 60%. This ensures that all the products were recovered, transferred over, and filtered. In addition to having the expected theoretical yield, the melting range was observed to be 114-115C which is a bit low compared to that reported in the lab manual, being 122C. This might be due to contaminants or perhaps the products not being completely dried. In addition, the melting range could be due to the heating apparatus heating up too fast thus the sample recovered wasn’t entirely pure and could have contained biphenyl. Overall, the experiment was conducted without any issues and the results were as expected. There were concerns about the experiment due to the specific requirements the experiment required to be executed without complications. For instance, the presence of water would have reacted with phenylmagnesium bromide which in turn would ruin the reaction. Also, excess heat could have affected the outcome leading to the production of biphenyl. However, the % yield and melting range verified that the experiment was conducted properly in accordance to the lab manual.