homework_#42_52
advertisement
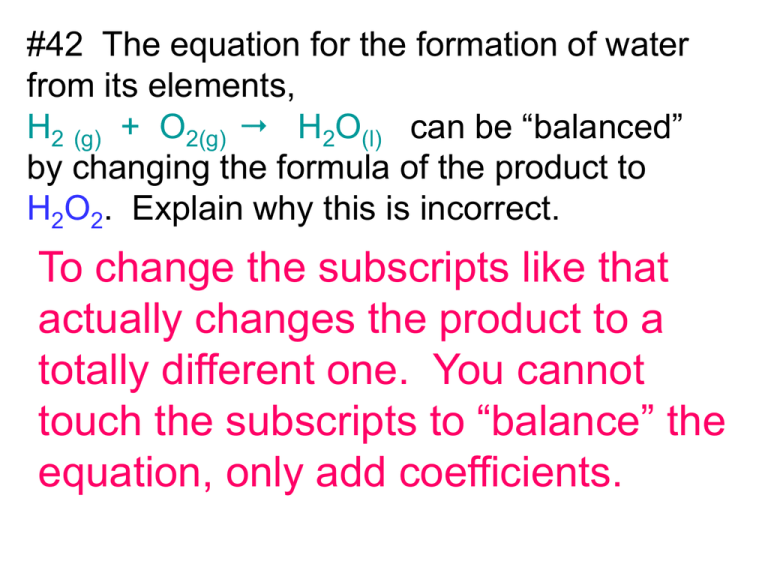
#42 The equation for the formation of water from its elements, H2 (g) + O2(g) H2O(l) can be “balanced” by changing the formula of the product to H2O2. Explain why this is incorrect. To change the subscripts like that actually changes the product to a totally different one. You cannot touch the subscripts to “balance” the equation, only add coefficients. #43. Balance the following equations: a. 2 PbO2 2 PbO + O2 b. 2 Fe(OH)3 c. Fe2O3 + 3 H2O (NH4)2CO3 2 NH3 + H2O + CO2 d. 2 NaCl + H2SO4 Na2SO4 + 2 HCl #44 What is a characteristic of every combination reaction? Every combination reaction has a single product. example: 2H2 + O2 2H2O single product #45 Write balanced chemical equations for the following combination reactions. a. 2 Mg + O2 2MgO b. P + O2 diphosphorous pentoxide 4P + 5O2 2P2O5 c. Ca + S CaS #46 What is a distinguishing feature of every decomposition reaction? a decomposition always has a single reactant. example: 2H2O 2H2 + O2 #47 Write a balanced chemical equation for each decomposition reaction: a. 2 Ag2O(s) 4 Ag b. + O2 ammonium nitrate dinitrogen monoxide NH4NO3 N2O + water + 2H2O #48 Use the activity series of metals to write a balanced equation for each single-replacement reaction. a. b. Au(s) NO reaction. K is higher on the chart than Au + KNO3 Zn(s) + 2AgNO3 Zn(NO3)2(s) c. 2 Al(s) + 3H2SO4 Al2(SO4)3 +2Ag + 3 H2 #49 write a balanced equation for each of the following doublereplacement reactions. a. H2C2O4(aq) + 2KOH(aq) 2H2O b. + K2C2O4 CdBr2(aq) + Na2S(aq) CdS + 2NaBr #50 What is common to all combustion reactions? oxygen on the left side example: C4H8 + 6O2 4CO2 + 4H2O #51 Write a balanced equation for the complete combustion of each compound. a. Butene (C4H8) C 4H 8 + 6O2 4CO2 + 4H2O b. acetone (C3H6O) C 3H 6O + 4O2 3CO2 + 3H2O #52 Balance each equation and identify its type. a. 3 Hf + 2 N2 Hf3N4 a combination reaction b. Mg + H2SO4 MgSO4 + H2 a single-replacement reaction c. 2 C2H6 + 7 O2 4 CO2 +6 H2O a combustion #52 Balance each equation and identify its type. continued…. d. Pb(NO3)2 + 2 NaI PbI2 + 2 NaNO3 a double-replacement reaction e. 4 Fe + 3 O2 2 Fe2O3 a combination reaction