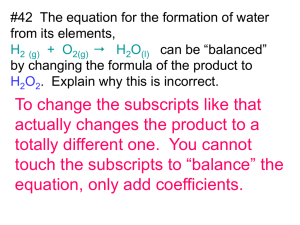
1405- practice Questions -chp 7.doc
advertisement

1405 Practice Questions Chapter 7 – Chemical reactions 1. Which of the following represents a balanced chemical equation? a. 2HCl + NaOH H2O + NaCl b. C2H2 + O2 2CO2 + H2O c. Na + H2O NaOH + H2 d. 6CO2 + 6 H2O C6H12O6 + 6O2 2. The reaction for photosynthesis is: 6CO2 + 6H2O C6H12O6 + 6O2 How many oxygen molecules (O2) are produced in the chemical reaction above? a. 1 b. 2 c. 6 d. 12 3. Which of the following is a true statement? a. Matter is conserved in a chemical reaction b. Matter is created in a chemical reaction c. Matter is destroyed in a chemical reaction d. All of the above statements can be true depending on the reaction 4. Which of the following equations is balanced? a. H2SO4 + 2NaOH Na2SO4 + H2O b. CH4 + Cl2 CH2Cl2 + HCl c. H2O + MgO Mg(OH)2 d. Al(OH)3 + H3PO4 AlPO4 + 2H2O 5. ___AlCl3 + ___Ba(OH)2 ___Al(OH)3 + ___BaCl2 When this equation is correctly balanced, the coefficient of the AlCl3 will be… a. 1 b. 2 c. 4 d. 6 6. ___H2O ___ H2 + ___O2 The coefficients of the correctly balanced equation for the reaction illustrated above are… a. 1,1,1 b. 1,1,2 c. 2,1,2 d. 2,2,1 7. A balanced chemical equation has equal numbers of atoms of each type on both sided of the equation. This illustrates the example of…. a. Conservation of energy b. Conservation of mass c. Action and reaction d. Natural selection 8. In chemistry class, Mr. Smoak adds a small piece of sodium metal to a glass of water. The sodium reacts violently with the water producing a small flame. The end products are hydrogen gas and sodium hydroxide. Which of the following is the correct balanced chemical equation for the reaction described above? a. 2Na + 2H2O 2NaOH + H2 b. Na + H2O + O2 3NaOH + H2 c. H2O2 + 2 Na 2NaOH + H2 d. 2NaOH + H2 2Na + 2H2O 9. When 127 g of copper reacts with 32 g of oxygen gas to form copper (II) oxide, no copper or oxygen is left over. How much copper (II) oxide is produced? a. 32 g b. 95 g 2Cu + O2 2CuO c. 127 g d. 159 g 10. In writing a chemical equation that produces hydrogen gas, the correct representation of hydrogen gas is ____. a. . H. b. 2H. c. H2. d. OH. Use the picture above 11. According to the law of conservation of mass, how much zinc was present in the zinc carbonate? a. 40 g b. 88 g c. 104 g d. 256 g 12. Which of the following reactions is an example of a single-replacement reaction? a. 2AgNO3 + Cu Cu(NO3)2 + 2Ag b. NaOH + HCl NaCl + H2O c. CO2 C + O2 d. 4Fe(OH)2 + O2 4Fe(OH)3 13. Which of the following reactions is a decomposition reaction? a. S8 + 8O2 8SO2 b. O2 + 2H2O 2H2O2 c. 2KClO3 2KCl + 3O2 d. 2Na + 2AgCl 2NaCl + 2Ag 14. Which is an example of a synthesis reaction? a. HCl + KOH KCl + H2O b. Pb(NO3)2 + 2HBr PbBr2 + 2HNO3 c. C + O2 CO2 d. Mg + H2SO4 MgSO4+ H2 15. What type of reaction does this illustration represent? a. Synthesis b. Decomposition c. Single Replacement d. Double Replacement 16. The following reaction is an example of __________? C2H6 + O2 CO2 + H2O a. Double replacement b. Single replacement c. Combustion d. Synthesis 17. Choose the correct classification and set of coefficients considering the following reaction: __KI + __PbCrO4 __K2CrO4 + __ PbI2 a. 2,1,1,1 and single replacement b. 2,1,1,1 and double replacement c. 1,1,2,1 and double replacement d. 1,1,2,1 and single replacement 18. Iron (III) chloride + Sodium carbonate Iron (III) carbonate + Sodium chloride Which of these is the balanced equation for the reaction described above? a. FeCl3 + Na2CO3 Fe2(CO3)3 + NaCl b. 2FeCl3 + 3Na2CO3 Fe2(CO3)3 + 6NaCl c. 2FeCl3 + 2Na2CO3 + 3Fe2(CO3)3 + 3NaCl d. 3FeCl3 + 2Na2CO3 3Fe2(CO3)3 + 6NaCl 19. Which coefficients correctly balance the formula equation NH4NO2 → N2 + H2O? a. 1, 2, 2 b. 1, 1, 2 c. 2, 1, 1 20. What are the missing coefficients for the skeleton equation below? Al2 (SO4)3 + KOH → Al(OH)3 K2SO4 a. 1, 3, 2, 3 b. 2, 12, 4, 6 c. 4, 6, 2, 3 d. 1,6, 2,3 d. 2, 2, 2 1405 Practice Questions- answers Chapter 7 – Chemical reactions 1. D 2. C 3. A 4. C 5. B 6. D 7. B 8. A 9. D 10. C 11. C 12. A 13. C 14. C 15. B 16. C 17. B 18. B 19. B 20. d Balance and write a balanced equation, ionic equation& net ionic equation the following reactions. 1. The reaction between sodium carbonate and silver nitrate: