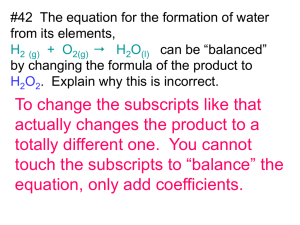
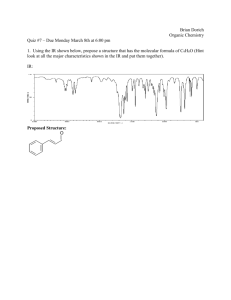
Microsoft PowerPoint 2007
advertisement

Studying Ozonolysis Reactions of 2-Butenes Using Cavity Ring-down Spectroscopy Liming Wang, Yingdi Liu, Mixtli Campos-Pineda, Chad Priest and Jingsong Zhang Jet Propulsion Laboratory, California Institute of Technology Department of Chemistry, University of California, Riverside VOCs: Alkanes, Alkenes, … 1 O3, PAN, HNO3, … Particles NOx VOCs: Alkanes, Alkenes, … + O3, PAN, HNO3, … Particles + NOx OH production mechanism in alkene + O3 reactions RH, hydrocarbon OH OH HONO +hv Alkenes O3 R, alkyl radical HO 2 ROOH OH carbonyl + alcohol O2 ROONO RO 2 NO RO 2 RO O2 NO 2 2 NO 2 RONO 2 hv Ozonolysis of Alkenes Reactions • • • • • • • Important oxidation pathway of alkenes in troposphere – High concentrations of O3 and alkenes in polluted areas Secondary organic aerosol (SOA) production in ozonolysis of large alkenes Production of OH radical (10-90% yield) and a source of HOx radical Production of Criegee intermediate (CI) CI react with many important molecules in the atmosphere : NO2, SO2, H2O etc OH production mechanism is not completely established Lack of kinetics information of CI Mechanisms of trans-2-Butene + O3 O O H3C HC O O3 CH O C H3C CH3 H3C C H CH3 H O Primary Ozonide Co-product of OH in the decomposition of Criegee intermediate O H3 C . H C 2 O O O syn O anti C C H 3C C O H H H H3C Criegee Intermediates Dioxirane HO O CH O O H H2C OH H CH C C H2 H (TS) Atkinson, Paulson, Donahue, Anderson, Marston, Cremer, and many others. Our Focus • OH production mechanism • By detecting co-product of OH using CRDS • CH2CHO from (trans/cis)-CH3CH=CHCH3 + O3 Cavity Ring-Down Spectroscopy Measure Rate of intensity decay instead of Magnitude of attenuation L Iin Iout ls Detector High-reflectivity Mirrors R 99.99 % A Wavelength Time profile Signal Processing Intensity Spectrum B A B Time Reference CRDS Spectrum of Vinoxy Radical . H C O C H H CH2CHO 305 310 315 320 325 330 335 340 345 350 Wavelength / nm Vinoxy radical was produced from photolysis of ethyl vinyl ether precursor. L. Wang et al. trans-2-Butene + O3 [CH2CHO] ~3 1011 molecule cm-3 HCHO Pressure Dependence of CH2CHO Production trans-2-butene and O3 in N2 total pressure Yields of CH2CHO decrease with increased total pressure. 8 Torr (a) 9.5 Torr (b) 12 Torr (c) Possible Reasons: 15.5 Torr (d) Increased CH2CHO depletion by O2; 19 Torr (e) 37 Torr (f) 65 Torr (g) 346.8 347.0 347.2 347.4 347.6 Wavelength (nm) 347.8 348.0 11 Kinetics model No. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Rate constants units: first Reaction C4H8 + O3 = CH3CHOO + CH3CHO C4H8 + O3 = OH + CH2CHO + CH3CHO; C4H8 + O3 = CH2CO + H2O + CH3CHO; C4H8 + O3 = CH3OH + CO + CH3CHO; C4H8 + O3 = CH4 + CO2 + CH3CHO; C4H8 + O3 = CH3CHO + other products; C4H8 + O3 = CH3CHO + OH + other products; CH2CHO + O2 = (CHO)2 + OH CH2CHO + O2 = HCHO + CO + OH CH2CHO + O2 = others OH + O3 = HO2 + O2 OH + C4H8 = others CH3OH + ·OH → (·)CH2OH + H2O CH3OH + ·OH → CH3O + H2O CH2OH + O2 = HCHO + HO2 HCHO + ·OH → HCO + H2O OH + CH2CHO = other products; CH3CHO + OH = CH2CHO + H2O CH3CHO + OH = H2O + CH3CO CH2CHO = other products; CH2CHO = WALL; C4H8 + CH2CHO = other products; CH3CHOO + C4H8 = P; CH3CHOO + O3 = P; CH3CHOO + CH3CHO = SOZ; CH3CHOO = OH + CH2CHO; CH3CHOO = WALL; CH3CHOO + HCHO = SOZ2; CH3CHOO + CH2CHO = P; order: s-1; second order: cm3 Branching ratio 0 0.5 0.05 0.07 0.11 0.27 0 0.1 0.3 0.6 0.85 0.15 0.05 0.95 Rate const 0 5.7E-17 9.5E-18 1.33E-17 2.09E-17 8.93E-17 0 6.12E-15 1.836E-14 3.672E-14 1.6E-12 6.4E-11 7.735E-13 9.1E-12 1E-11 1E-11 7.5E-13 0 10 0 1E-15 1E-13 1E-12 76 10 1E-12 1E-11 molecule-1 s-1; third order: cm6 molecule-2 s-1 Kinetics model 29 reactions 5 13 Concentration by CRDS (10 ) No. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 4 3 2 1 0 0 Rate constants 1 Reaction C4H8 + O3 = CH3CHOO + CH3CHO C4H8 + O3 = OH + CH2CHO + CH3CHO; C4H8 + O3 = CH2CO + H2O + CH3CHO; C4H8 + O3 = CH3OH + CO + CH3CHO; C4H8 + O3 = CH4 + CO2 + CH3CHO; C4H8 + O3 = CH3CHO + other products; C4H8 + O3 = CH3CHO + OH + other products; CH2CHO + O2 = (CHO)2 + OH CH2CHO + O2 = HCHO + CO + OH CH2CHO + O2 = others OH + O3 = HO2 + O2 OH + C4H8 = others CH3OH + ·OH → (·)CH2OH + H2O CH3OH + ·OH → CH3O + H2O CH2OH + O2 = HCHO + HO2 HCHO + ·OH → HCO + H2O OH + CH2CHO = other products; CH3CHO + OH = CH2CHO + H2O CH3CHO + OH = H2O + CH3CO CH2CHO = other products; CH2CHO = WALL; C4H8 + CH2CHO = other products; CH3CHOO + C4H8 = P; Vinoxy CH3CHOO + O3 = P; HCHO CH3CHOO + CH3CHO = SOZ; CH3CHOO = OH + CH2CHO; CH3CHOO = WALL; 5 10 15 20 25 30 CH3CHOO + HCHO = SOZ2; CH3CHOO + CH2CHO = P; units: First order: s-1; Second order: cm3 molecule-1 Time/s Branching ratio 0 0.5 0.05 0.07 0.11 0.27 0 0.1 0.3 0.6 0.85 0.15 0.05 0.95 Rate const 0 5.7E-17 9.5E-18 1.33E-17 2.09E-17 8.93E-17 0 6.12E-15 1.836E-14 3.672E-14 1.6E-12 6.4E-11 7.735E-13 9.1E-12 1E-11 1E-11 7.5E-13 0 10 0 1E-15 1E-13 1E-12 76 10 1E-12 1E-11 s-1; Third order: cm6 molecule-2 s- 6E+11 Vinoxy Conc (molecules/cm3) Exp Vinoxy (mol/cm^3) Sim: a=0.5 5E+11 Sim: a=0.3 4E+11 3E+11 2E+11 1E+11 CH2CHO yield (a) is 0.3-0.5 0 5.0 10.0 15.0 20.0 Pressure/Torr Pressure dependence study of simulation when a= 0.3 and a =0.5 and experimental results. 14 Summary CH2CHO is observed from 2-butene ozonolysis reactions: –CH2CHO + OH is a considerable channel; –Chemical kinetic modeling of the vinoxy and formaldehyde production indicates that the CH2CHO yield is 0.3-0.5 and 0.2-0.3 in the ozonolysis reaction of trans- and cis-2butene, respectively. –The CH2CHO yields are consistent with the OH yields of trans- and cis-2-butene. 15 Acknowledgement National Science Foundation $$ Keck Foundation $$ Mixtli Campos-Pineda Chad Priest Prof Jingsong Zhang Prof Liming Wang