FeSCN_presentation
advertisement
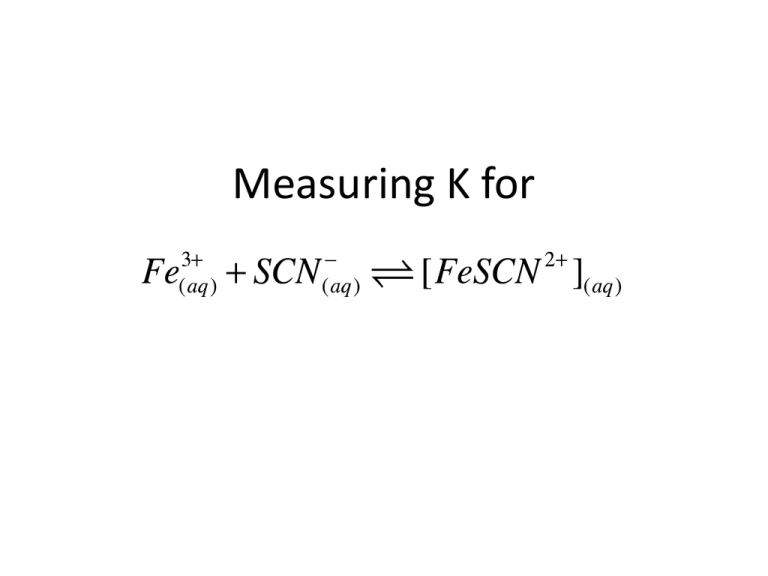
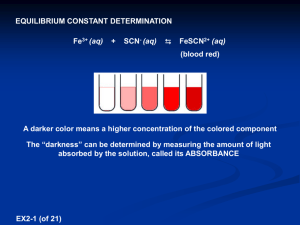
Measuring K for The Reaction • But we only want • Make sure [Fe3+]o >> [SCN-]o The Reaction • Fe3+ can be very insoluble because it can act as a Lewis Acid • Drive equilibrium left by adding HNO3(aq) Measuring Equilibrium Concentrations Deep red color • The complex absorbs in the blue/green/yellow, that is why it is red • We can monitor the concentration of the product using Beer’s Law Beer’s Law “The stronger the brew, the less light that goes through” A = e ×l × éë FeSCN 2+ ùû Path Length Absorbance Molar Extinction Coefficient 𝐴 = log 𝐼0 𝐼𝑡 Beer’s Law A = e ×l × éë FeSCN 2+ ùû • If we know and l we can measure the concentration by measuring the absorbance • We can do this by calibrating the spectrometer, by measuring the absorbance at known concentrations of [FeSCN2+] • The slope of the calibration curve is ε.l Part A: Calibrating the Spectrometer • But hang on how can we get known [FeSCN2+]eq to calibrate? • Add a LOT of Fe3+, this will drive the reaction to completion (Le Chatelier’s Principle) • Then [SCN-]o ≈ [FeSCN2+]eq Part A: Calibrating the Spectrometer Flask # 0.200 M Fe(NO3)3 (mL) 0.00200 M KSCN (mL) 0.1 M HNO3 (mL) 1 5.00 0.00 20.00 2 5.00 5.00 15.00 3 5.00 4.00 16.00 4 5.00 3.00 17.00 5 5.00 2.00 18.00 6 5.00 1.00 19.00 Pipette the reactants into a 25mL volumetric flask, and fill up to the calibration mark on the flask with the 0.1M HNO3 Part A: Calibrating the Spectrometer Flask # 0.200 M Fe(NO3)3 (mL) 0.0020M KSCN (mL) 0.1 M HNO3 (mL) 1 5.00 0.00 20.00 2 5.00 5.00 15.00 3 5.00 4.00 16.00 4 5.00 3.00 17.00 5 5.00 2.00 18.00 6 5.00 1.00 19.00 [Fe3+]o [SCN-]o [FeSCN2+] ≈ [SCN-]o • Calculate the initial concentrations [Fe3+]o, [SCN-]o = [FeSCN2+]eq • Measure A for each sample [FeSCN 2+ ]eq » [SCN - ]o = 0.002M × [Fe3+ ]o = 0.200M × Abs VSCN 25.00mL 5.00mL = 0.04 M 25.00mL Part A: Calibrating the Spectrometer • Calibrate the SpectroVis spectrometer with distilled H2O filling a cuvette • Measure the absorbance spectrum of FeSCN2+ • Identify the wavelength of maximum absorption lmax • Conduct a Absorbance vs. Concentration experiment on the SpectroVis by selecting and choosing Abs vs Concentration • Get slope of A vs. [FeSCN2+] curve = m Part B: Measuring Kc 𝐹𝑒𝑆𝐶𝑁 2+ 𝑒𝑞 𝐹𝑒𝑆𝐶𝑁 2+ 𝑒𝑞 𝐾𝐶 = 𝐹𝑒 3+ 𝑒𝑞 × 𝑆𝐶𝑁 − 𝐴 = 𝑚 𝐾𝐶 = 𝐹𝑒 3+ 𝐴/𝑚 − 0 − 𝐴/𝑚 × 𝑆𝐶𝑁 We can get Kc values from 2-6 they should all be close to each other 0 𝑒𝑞 − 𝐴/𝑚