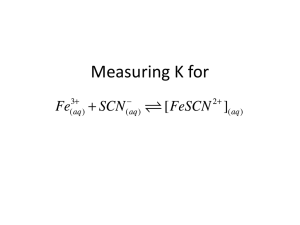data sheets
advertisement

LAB: The Determination of Keq for FeSCN2+ (30 points possible) Pre-lab Questions: (5 pts) 1) 2a) Ag+(aq) 2b) + 2NH3(aq) → Ag(NH3)2+(aq) I C . E 3) 4) Reference Solutions #1-5: Calculate initial [SCN-] for each reference solution (#1-5) and enter the values in Data Table 1 where indicated. The background section explains why [SCN-]initial ≈ [FeSCN2+]eq’m for these five reference solutions. Show a sample calculation for Ref sol’n #1 in this space. Work: #1 Answer: #1 [SCN-]initial = 5) Test Solutions #6-10: Calculate initial [Fe3+] and [SCN-] for each reference solution (#6-10) and enter the values in Data Table 2 where indicated. Show a sample calculation for Ref sol’n #6 in this space. Work: #6 - Show for both [Fe3+] and [SCN-] Answer: #6 [Fe3+]initial = #6 [SCN-]initial = Data Tables (10 points) Data Table 1 – Reference Solutions Temperature: [FeSCN2+] Sample Absorbance Same as initial [SCN-], calculated in #4 of prelab Reference solution #1 Reference solution #2 Reference solution #3 Reference solution #4 Reference solution #5 Data Table 2 – Test Solutions Temperature: Sample [Fe3+]initial* [SCN-]initial* Calculated in #5 of prelab Calculated in #5 of prelab Absorbance Test solution #6 Test solution #7 Test solution #8 Test solution #9 Test solution #10 *These are the concentrations of ions in solution immediately after mixing and before any reaction has occurred. Data Table 3 - Results Sample [FeSCN2+]eq [Fe3+]eq [SCN-]eq Keq Determined from linear trendline eq’n on graph Test solution #6 Test solution #7 Test solution #8 Test solution #9 Test solution #10 Average Keq:_____________ Average deviation:___________ Post-lab Calculations and Analysis (13 points) 1) Attach graph (should be size of full page and either printed from Excel (with trendline and equation) or plotted on graph paper with equation of line determined via your calculator). Use the equation of trendline to determine equilibrium concentrations of your test solution instead of visual approximations from the graph (#2-3 below). Don’t forget to title your graph and label each axis appropriately. (5 pts) 2-3) Record results in Data Table 3 (1 pt) (show work for test sol’n #6 as a sample calculation by plugging #’s into eq’n from graph) 4) Sample calculation for test solution #6: (3 pts) Complete the I-C-E chart below to find the eq’m concentrations of Fe3+(aq) and SCN-(aq) instead of using the eq’n provided in the lab. Fe3+(aq) + SCN-(aq) → FeSCN2+(aq) I C E . ____________ ____________ 6) Sample Keq calculation for test solution #6: (1 pt) 7) Record work under data table 3 (no need to show work) 8) Show work for calculation of average deviation of Keq: (1 pt) 9) (1 pt) 10 Error #1 (1 pt) #1 Effect? Keq ↑ or ↓ or random #2 (1 pt) #2 Effect? Keq ↑ or ↓ or random #3 (1 pt) #3 Effect? Keq ↑ or ↓ or random Post-lab Question: (2 pts) Answer question # 4 only: #4)