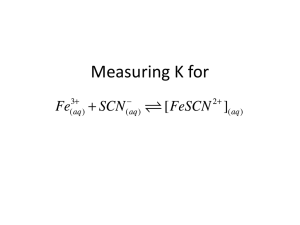Determination of the Equilibrium Constant
advertisement

Determination of the Equilibrium Constant Kyle Miller December 11, 2006 1 Purpose The purpose of this experiment is to determine the equilibrium constant for the reaction Fe3+ + SCN− FeSCN2+ and to see if the constant is indeed the same under different conditions. 2 Procedure First, reference solutions are made by mixing an excess of Fe3+ ions with known amounts of SCN− ions. We then assume that the reactions are driven to completion due to Le Chtelier’s Principle, so they contain a known concentration of FeSCN2+ ions. Second, test solutions are made by mixing a constant amount of Fe3+ ions with varying amounts of SCN− ions, which contain an unknown concentration of FeSCN2+ ions. Then, the absorbance of the solutions are measured with a spectrophotometer. With the reference solution’s absorbances, a calibration curve is made to then determine the concentrations of the test solutions. Then, with the calculated concentrations, the equilibrium constant can be calculated. 3 Data The following data were collected: 1 3.1 Reference Solutions Sample Reference #1 Reference #2 Reference #3 Reference #4 Reference #5 3.2 Absorbance 0.233 0.299 0.409 0.524 0.600 Test Solutions Sample Test #6 Test #7 Test #8 Test #9 Test #10 4 [FeSCN2+ ] 4.0 × 10−5 6.0 × 10−5 8.0 × 10−5 1.0 × 10−4 1.2 × 10−4 [Fe3+ ] 1.0 × 10−3 1.0 × 10−3 1.0 × 10−3 1.0 × 10−3 1.0 × 10−3 [SCN− ] 2.0 × 10−4 4.0 × 10−4 6.0 × 10−4 8.0 × 10−4 1.0 × 10−3 Absorbance 0.233 0.412 0.568 0.792 0.985 Calculations Using the method of least squares with the absorbance of the reference solutions, we can find that A = 5010 · [FeSCN2+ ] + 0.011 (1) Where A is the absorbance for each [FeSCN2+ ]. This curve can also be written for the concentration. A − 0.011 [FeSCN2+ ] = (2) 5010 Using this equation on each test solution absorbance, we can find the unknown [FeSCN2+ ]eq concentrations. For example, with the absorbance of 0.233, the [FeSCN2+ ]eq = 0.233−0.011 = 5010 −5 4.4 × 10 Since each compound in the reaction is used or made in a 1:1 ratio, they will decrease or increase (respectively) by the same amount. So, let [FeSCN2+ ] increase by x, then [Fe3+ ] will decrease by x. Then, since [FeSCN2+ ] is starting at 0 for the experiment, the x is the final concentration at equilibrium. For example, if [FeSCN2+ ] gets to be 4.4 × 10−5 , [Fe3+ ] will be 1.0 × 10−3 − 4.4 × 10−5 = 9.6 × 10−4 With the same logic, we can find [SCN− ]eq which is the initial concentration of SCN− less the change in [FeSCN2+ ]. This is, for example, 2.0 × 10−4 − 4.4 × 10−5 = 1.6 × 10−4 2 According to known rules, the equilibrium constant for this reaction is Kp = [FeSCN2+ ] [Fe3+ ][SCN− ] (3) Then, using calculations from the results table, we can find the Keq values for each test −5 solution. For example, for test solution #6, the Keq = (9.6×104.4×10 −4 )(1.6×10−4 ) = 290 The mean of the Keq values is P Keq 5 The average deviation of the Keq is 4.1 = 280 P |Keq −280| 5 = 16 Results Sample Test #6 Test #7 Test #8 Test #9 Test #10 [FeSCN2+ ]eq 4.4 × 10−5 8.0 × 10−5 1.1 × 10−4 1.6 × 10−4 1.9 × 10−4 [Fe3+ ]eq 9.6 × 10−4 9.2 × 10−4 8.9 × 10−4 8.4 × 10−4 8.1 × 10−4 [SCN− ]eq 1.6 × 10−4 3.2 × 10−4 4.9 × 10−4 6.4 × 10−4 8.1 × 10−4 Keq 290 270 250 300 290 Mean: 280 Average Deviation: 16 5 Discussion 1. The equilibrium constant is a quantity which characterizes an equilibrium in a reaction and is based on the final concentrations of involved compounds. The value was constant for all of the experiments (within a good margin of error). It should be consistent because this constant is defined to be the relation between all concentrations of involved compounds at chemical equilibrium. 2. The calculated value of the equilibrium constant indicates that there are mostly products since it is 280 > 1. Also, since 280 is quite large (compared to the Haber process which has a K of about 30), it should prefer the products. 3. A spectrophotometer is a device that measures the amount of light that can pass through a given substance. In this experiment, we used it to determine the percent absorbance for each solution. The reference solutions were obtained by mixing a known amount of SCN− in an excess of Fe3+ so that we could assume that the reaction went to completion and we would have a known amount of FeSCN2+ . We can then find the absorbances of the 3 reference solutions to create a function that relates concentration to absorbance which can then be applied to the unknown concentrations with the measured absorbances to calculate the unknown concentration. 4. The spectrophotometer should not be set to the same color as that of the solution because the visible color is that which is transmitted which mean none is absorbed. Another color’s absorbance, such as the complement of the solution’s color, such as cyan (since the complex ion is a red), or the components of cyan (green and blue), would probably change with different concentrations because it is a color that must be absorbed to only allow red through. In this experiment, 450nm light was used, which corresponds to blue. Since the complex ion is red, this blue light will be absorbed and can then be measured. 5. The precision indicates that the equilibrium constant is indeed constant for the experi16 ment because it is such a small percentage of the mean Keq . It is only 280 = 5.7% of the mean constant. This means that the calculated Keq for each solution is very close to each other and that, for allowing for experimental error, they really are equal (noting that they seem to be randomly larger or smaller than the mean without any correlation to any of the concentrations that could otherwise explain this behavior). 4