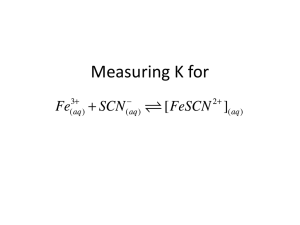CHEMISTRY 12 : EQUILIBRIUM MINILAB

CHEMISTRY 12 : EQUILIBRIUM MINILAB
In this "quick –and–dirty" experiment, we will study the reaction: Fe 3+ (aq) + SCN
–
(aq)
FeSCN 2+ (aq) .
The FeSCN 2+ is red in aqueous solutions.
Procedure and Observations and Analysis and Everything :
1. Mix about 25 mL of 0.002 M KSCN and 25 mL of water in a beaker.
Description of the solution: ________________________________________________
The ions present in KSCN(aq) are: ___________________________________________
Obtain a solution of 0.2 M Fe(NO
3
)
3
.
Description of the solution: ________________________________________________
The ions present in Fe(NO
3
)
3
(aq) are: ________________________________________
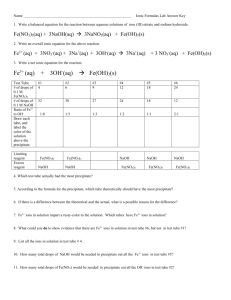
2. Add 5 drops of the Fe(NO
3
)
3
solution to the beaker, and mix.
Description of the solution: ________________________________________________
What TWO pairs of ions might be responsible for the colour change after adding Fe(NO
3
)
3
?
_____________________________________________________________________
3. Observe a solution of KNO
3
.
Description of the solution: ________________________________________________
Could the ions present in KNO
3
be responsible for the observations in #2? Why?
____________________________________________________________________
4. Considering your answer to #3, what pair of ions MUST be responsible for the change noted in #2?
________________________________
5. Divide the beaker contents equally among 4 evaporating dishes. Save the solution in one of the dishes to act as a reference for colour comparison purposes.
6. To one of the dishes add a few crystals of solid KSCN. Observe, stir and observe again.
Observations: __________________________________________________________ a) What effect did the addition of KSCN(s) have on the [SCN – ] in solution after stirring?
_________________________________________________________________ b) What VISIBLE effect did the addition of KSCN(s) have on the [FeSCN 2+ ]? (FeSCN 2+ is the red ion produced in the reaction.)
_________________________________________________________________ c) Was Fe 3+ present in excess before you added the KSCN(s)? How do you know?
_________________________________________________________________
_________________________________________________________________
Page 2
7. Add an extra 3 drops of Fe(NO
3
)
3
to another evaporating dish of solution.
Observations: _________________________________________________________ a) What effect did the addition of Fe(NO
3
)
3
have on the [Fe 3+ ] in solution after stirring?
_________________________________________________________________ b) What effect did the addition of Fe(NO
3
)
3
have on the [FeSCN 2+ ]?
_________________________________________________________________ c) Was SCN
–
present in excess before you added the Fe(NO
3
)
3
? How do you know?
__________________________________________________________________
__________________________________________________________________
8. Compare what you wrote for #6(c) and #7(c). Suggest how you might account for these results.
9. Add a few crystals of Na
2
HPO
4
to another evaporating dish of liquid. Observe for a minute and
2 – then stir.
Fact: Fe 3+ and HPO
4
form an almost completely insoluble solid having a translucent white colour.
Observations: __________________________________________________________
2 – a) What effect does the addition of HPO
4
have on the [Fe 3+ ] IN SOLUTION?
_________________________________________________________________
2 – b) What effect does the addition of HPO
4
have on the [FeSCN 2+ ]?
_________________________________________________________________ c) What would have to be true about the reaction: Fe 3+ + SCN –
FeSCN 2+ in order for your statements in #9(a) and (b) to be make sense?
_________________________________________________________________
Let’s summarize our findings: a) What happens to the concentration of the product if you increase the concentration of a reactant?
If a reaction starts at equilibrium, which side will it "shift" to if the concentration of a reactant is increased? b) What happens to the concentration of the product if you decrease the concentration of a reactant?
If a reaction starts at equilibrium, which side will it "shift" to if we decrease the concentration of a reactant?