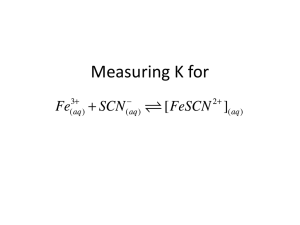FeSCN+2
advertisement

Determination of Formation Constant, Kf of Thiocyanoiron(III), FeSCN+2 Dr. Fred Omega Garces Chemistry 201 Miramar College Chemical Equilibrium: Finding the Formation Constant of FeSCN2+ (aq) Fe3 +(aq) iron(III) + SCN–(aq) FeSCN2+(aq) D thiocyanate thiocyanoiron(III) kf = € FeSCN2 + [ ] Fe +3 [SCN− ] [ ] Objective The purpose of this experiment is to determine the constant formation, Kf, (equilibrium constant) for the formation of thiocyanoiron(III). Fe3+ (aq) + SCN-(aq) Kf D FeSCN2+ (aq) Background Information Consider the following reaction: Fe3+ (aq) + SCN-(aq) D FeSCN2+ (aq) The mass action expression iskf = [FeSCN2+ ] eq [Fe 3+ ] eq [SCN- ] eq To determine the equilibrium constant, the equilibrium concentration of [Fe3+]eq , [SCN-] eq € [FeSCN2+] will need to be known. and eq Chemcials Fe3+ from Fe(NO3)3, yellowish SCN- from kSCN, colorless FeSCN2+ from kSCN, dark red absorbs blue λmax =447nm According to Beer's Law, A = ε l [c] so the AFeSCN can be used to determined [FeSCN2+] eq . 2+ How can the concentrations of [Fe3+]eq and [SCN-] eq be determined by simply monitoring the [FeSCN2+] eq absorbance? Chemical Reaction Initial Concentration of Fe3+ is known as well as the initial concentration of from SCN- . Furthermore, the reaction is carried out so that the [Fe3+]i >> [SCNi , this ensures that the product [FeSCN2+] depends on the [SCN-] i . Fe3+ Excess + SCN Limiting ! FeSCN2+ Amount produced depends on limiting [SCN-] How can the concentrations of [Fe3+]eq and [SCN-] eq be determined 2+ €by simply monitoring the [FeSCN ] eq absorbance? Background Information -The equilibrium process. To determine Kf for FeSCN2+, all the concentrations of the specie in the reaction: Fe3 +(aq) + SCN–(aq) FeSCN2+(aq) D This can be done by investigating the reaction as the equilibrium is established. colorless colorless Fe3 +(aq) + SCN–(aq) i [Fe+3]i [SCN-] i Δ -x -x [e] [Fe+3] i -x Blood red [SCN-] D FeSCN2+(aq) 0 +x i - x [FeSCN2+] eq Background Information - Determining Concentration at Equilibrium [FeSCN2+] eq = x Determined by absorbance Therefore [SCN-] eq = [SCN-] i – x = [SCN-] i – [FeSCN2+] [Fe+3] eq = [Fe+3] i – x = [Fe+3] i - [FeSCN2+] eq eq Background Information - Determining Concentration of [FeSCN2+] trials [FeSCN2+] eq is red and absorbs blue light 470nm. Standard solution prepared with large Fe+3 concentration added to small amount of SCN-. This results in practically all the Fe+3 converting to SCN-. [Fe+3](large amount) 100 x + [SCN-] (small amount) D [FeSCN2+] 1x (same amount [SCN-] ) ~1 Astd g [FeSCN2+]std & for other rxns: Atrial# ∝ [FeSCN2+]trial# From Beer's Law A = ε b [FeSCN ] 2+ Atrial# 1 [FeSCN2+ ]trial# = Astd 1 [FeSCN2+ ]std € Therefore ⇒ [FeSCN2+ ]trial# = Atrial# ε b [FeSCN2+ ]trial# = , Astd ε b [FeSCN2+ ]std Atrial# [FeSCN2+ ]std Astd ….Background Information Mass Action Expression FeSCN ] [ = [Fe ][SCN ] [FeSCN ] [Fe ] • [SCN ] 2+ kf +3 2+ = − 3+ 3+ eq1 [ ] = Fe3+ i [ [ € #% 3+ Fe $ [ ] i [ - FeSCN2 + eq1 [FeSCN ] 2+ - FeSCN2 + ] [ = FeSCN2 + [SCN ] , eq1 eq1 - eq1 [ std • A1 std eq - i ] 2+ eq1 &( ' • std = SCN - ] A &(#% ] '$[SCN ] - [FeSCN ] FeSCN2 + kf = - eq1 At equilibrium for trial #1 : [Fe ] eq1 ] i A1 Astd [ - FeSCN2 + ] eq1 Pictorial Flowchart GogglesVenier with Colorimetry - Test tubes Volumetric Pipets Set up GogglesVernier with Colorimetry- Test tubes Volumetric Pipets Procedure Absorption Spectra Excel Data