PROTEIN FOLDING
advertisement
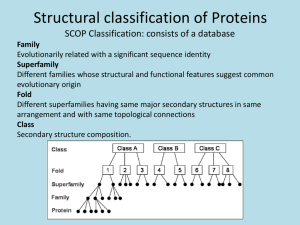
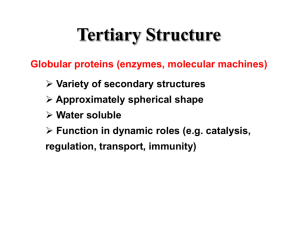
PROTEIN FOLDING • Major Question: Precisely how is the onedimensional sequence of a protein programmed to achieve a definitive threedimensional structure? The transition is from a random coil to a unique structure. • There are no definitive answers! There are, however, several important considerations. RNAase ►Single Chain of 124 residues. ►Stabilized by 4 disulfide bonds Ribonuclease Experiments • Disulfide bonds of RNAase reduced with βmercaptoethanol in 8M urea. • All activity lost; S-S bridges broken, converted to –SH. RESTORATION OF ACTIVITY • Oxidation allowed RNAase activity regained • Original, native conformation formed, with all correct S-S bonds reestablished. • Probability of correct S-S bonds by chance, 1%. CONCLUSIONS TO RNAase STUDIES • Native conformation of a protein is the state with the lowest Gibbs free energy. • Proteins follow unique paths to attain a native state. • Primary structure possesses sufficient information for proper folding. ENERGETICS OF FOLDING GENERAL PRINCIPLES OF THERMODYNAMICS ΔG = ΔH – TΔS “Goldfish are Hell without Tartar Sauce” Where: ΔG = free energy, energy available for work ΔH = enthalpy, a measure of bond formation (-) or a measure of bond breaking (+) T = temperature, oK ΔS = entropy, a measure of increasing chaos (+) or a measure of decreasing chaos (-) For a spontaneous process, ΔG must be (-) Levintal’s Paradox (1968) Reiteration of Anfinsen’s conclusions Small protein (100 AA’s) Each AA can assume 3 positions Total possible structures = 3100 = 5 x 1047 Examine each structure for 1 x 10-13s Then total search requires 5 x 1034s This is 1.6 x 1027 years, a period longer than the age of the universe! FOLDING OF GLOBULAR PROTEINS ΔG = ΔH - TΔS Since ΔG is (-), bond formation and/or an increase in disorder must predominate. Random coil breaks bonds with aqueous solvent (+ΔH), but forms new bonds internally (-ΔH). The enthalpy term is thus minimal. Thus, a (+ΔS) is the deciding factor which occurs as a result of the loss of structured water surrounding hydrophobic domains on the random coil. This type of folding is thus entropy driven. DATA SUPPORTING ENTROPY AS A DRIVING FORCE FOR FOLDING •Heat capacity, Cp, (cal/deg-mole) of protein solutions: on denaturation, Cp increases on refolding, Cp decreases Thus, the aqueous medium is more structured when the protein is denatured, and less structured when the protein is in its native conformation. •Addition of alcohols and similar reagents to protein solutions leads to protein denaturation. FOLDING OF FIBROUS PROTEINS For fibrous proteins, a “collapse” as described for globular proteins is prevented by the prevalence of polar amino acids seeking an aqueous environment plus a high content of amino acids for which large alterations in dihedral bond angles are difficult. Folding for these proteins is usually enthalpy driven. BOTTOM LINE ON FOLDING ΔG (folding) = -5 to -15 kcal/mole For a 100 AA protein, where ΔG = -10, stabilization/AA = 0.1 kcal/mole. This is less than random thermal motion, 0.6 kcal/mole! Significance: evolution has favored flexibility native proteins are on the borderline of denaturation misfolding is a common occurence Pathways of folding include the molten globule state. This slide shows the structure of the molten globule state (a) and the native, folded state (b) of cytochrome b562. The molten globule state is somewhat larger than the final native conformation and is readily detected by size exclusion chromatography. Molecular Chaperones • Why are chaperones needed if folding is inherent in the sequence? – to protect nascent proteins from the concentrated protein matrix in the cell – to accelerate slow steps – to ensure correct folding • Chaperone proteins were first identified as "heat-shock proteins" (hsp60 and hsp70) Protein folding pathways. (a) Chaperone-independent folding. (b) Hsp70-assisted protein folding. (c) Folding assisted by Hsp70 and chaperonin complexes. The chaperonin complex in E.coli is GroES-GroEL. The chaperonin complex in eukaryotic cells is known as TRiC (for TCP-1 ring complex) or CCT (cytosolic chaperonin-containing TCP-1). Structure and Function of the GroEL-GroES complex. (a) Space-filling representation and overall dimensions of GroEL-GroES (top view, left;side view, right). GroES is gold; the top , or apical, GroEL ring is green, and the bottom GroEL ring is red. (b) Section through the center of the complex to reveal the central cavity. The GroEL-GroES structure is shown as a Ca Carbon trace. ADP molecules bound to GroEL are shown as space-filling models. (c) Model of the GroEL cylinder (blue) in action. An unfolded (U) or partially folded (I) polypeptide binds to hydrophobic patches on the apical ring of a7-subunits, followed by ATP binding and GroES (red) association. ATP binding triggers a conformational change that buries the a7-subunit hydrophobic patches (yellow), releasing the polypeptide into the central activity (“Anfinsen cage”). After about 15 seconds, ATP hydrolysis takes place, followed by binding of ATP to the lower a7-subunit ring, which causes release of the protein. ADDITIONAL FACILITATORS 1. PDI – protein disulphide isomerase PDI-S + -S – S - PDI - S – S - + S – 2. PPI – peptidyl prolyl isomerase catalyzes cis-trans isomerization of X-P bonds. Ken Dill’s folding funnel. A good summary view. Unfolded structures lie around the top. As the protein folds, it falls down the wall of the energy funnel to more stable conformations. The native, folded structure is at the bottom. Nature Structural Biol. 4, 10-19 (1997). GENERATION OF ALZHEIMER PEPTIDE Amyloid precursor protein cleaved by two proteases to generate amyloid beta protein (A). PRION PRECURSOR PROTEIN Protein anchored to membrane of nerve cell. TRANSFORMATION OF PRION PROTEIN Advent of pleated sheet dominance