empirical formula
advertisement

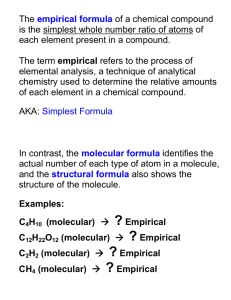
Empirical and Molecular Formulas % Composition The percent by mass of each element in a compound 42. ) 36.11% Ca; 63.89% Cl 43. ) 32.37% Na, 22.58%S, 45.05% O 44. ) H2SO3 45. ) 3.08% H, 31.61% P, 65.31% O Empirical Formula The formula with the lowest whole-number mole ratio of the elements EX: CH2O is the empirical formula glucose Molecular Formula Formula that specifies the actual # of atoms of each element in one molecule or formula unit of the substance EX: C6H12O6 is the molecular formula for glucose. Steps to Determine an Empirical Formula 1.) Find the # of moles of each element. (Divide the amount of each element by its molar mass.) 2.) Divide each mole value by the smallest answer. 3.) Round to the nearest whole #, if it is close. This is the mole ratio of the elements and is represented by subscripts in the empirical formula. **If you get ___.5 at this step, multiply EACH by 2. **If you get ___.25 at this step, multiply EACH by 4. **If you get ___.33 or ___.66, multiply EACH by 3. If you are given “%” of each element instead of a mass in grams, just replace the “%” with “g” and calculate as you normally do with “g”. Determining the Molecular Formula To determine the molecular formula of a compound, 1. Calculate the following: Experimentally determined molar mass mass of the empirical formula 2. Multiply that answer through the empirical formula molecular formula = (empirical formula)n Determining the Formula for a Hydrate 1. 2. 3. 4. 5. 6. Write down ALL info., including the mass of both the hydrated compound AND the anhydrate Calculate: hydrated cpd. - anhydrate = g H2O Find the moles of the anhydrate and the moles of H2O Get the mole to mole ratio (by dividing by the smaller number from #3 above) Write the formula, using a “ “ (dot) to attach the H2O Write the name of the hydrate Look at Example Problem 11-14 on page 340.