Moles, Empirical & Molecular Formulas Chemistry Review
advertisement

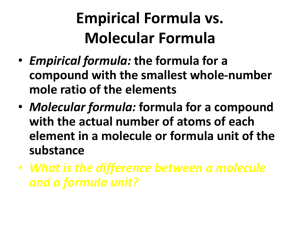
CP CHEMISTRY Moles Part 2 Review Name: ____________________________ ___ period 1. What is percent composition? 2. What is the percent composition of the elements in CaCl2? 3. What is the percent of oxygen in phosphoric acid, H3PO4? 4. What is the percent of phosphorus in barium phosphate, Ba3(PO4)2? 5. What is an empirical formula? 6. Determine the empirical formulas for the following compounds: a. ethylene – C2H4 b. ascorbic acid – C6H8O6 c. naphthalene – C10H8 7. What are the steps to determine a compound’s empirical formula? CP CHEMISTRY, Moles Part 2 Review, page 2 8. What is the empirical formula for MSG (monosodium glutamate) if it contains 35.5% C, 4.77% H, 8.29% N, 37.9% O, and 13.6% Na? 9. What is the empirical formula of a compound that contains 10.53 g Ni, 4.38 g C, and 5.10 g N? 10. What is the empirical formula for vanadium oxide if it contains 56.0% vanadium and 44.0% oxygen? CP CHEMISTRY, Moles Part 2 Review, page 3 11. What is a molecular formula? 12. What are the steps to determining a molecular formula? 13. What is the molecular formula of a lead compound which contains 59.37% lead and 40.63% chlorine, if the molar mass of the compound is determined to be 349.0 g/mol? CP CHEMISTRY, Moles Part 2 Review, page 4 14. Glycerol is a thick liquid containing 39.12% carbon, 8.75% hydrogen, and 52.12% oxygen. Its molar mass is 92.09 g/mol. What is its molecular formula? 15. What is a hydrate? 16. What are the prefixes used to name hydrates? 17. What are the steps to determine the formula of a hydrate? CP CHEMISTRY, Moles Part 2 Review, page 5 18. A mass of hydrated calcium sulfate is 4.89 g. It is heated and the water driven off leaving 3.87 g of anhydrous calcium sulfate. What is the formula of the hydrated? 19. A 1.628 g of hydrated magnesium iodide is heated until its mass is reduced to 1.072 g and all water has been removed. What is the formula of the hydrate?