Lab- Freezing Point

Science 8 Mrs. Cavanagh
Per_____
Name__________________________________
Date___________________________________
Activity: Freezing Point of a Substance
1.
Get- melted stearic acid in test tube, beaker, and thermometer.
2.
Keep tube in beaker. Keep thermometer in test tube. Do not remove.
3.
Measure and record temperature as soon as you sit down in chart below under time 0 (start).
4.
Keep recording every minute for 15 minutes.
5.
Make a line graph of the data on the graph provided.
11
12
13
14
15
7
8
9
10
Time (min) Temp. (°C)
0
1
2
3
4
5
6
Graph:
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
Time in minutes
Questions: Put answers on the side.
1.
What is the freezing point of stearic acid?
1.
About 15 C 3. About 20
2.
About 50 C 4. About 100 C
2.
What is the melting point of stearic acid?
1.
About 15 C
2.
About 50 C
3. About 20
4. About 100 C
3.
What was happening at the flat part of the graph?
1.
Stearic acid was freezing.
2.
Stearic acid was melting.
3.
Stearic acid was evaporating.
4.
Stearic acid was condensing.
Answers:
1.
2.
3.
4.
5.
6.
7.
_____
_____
_____
_____
_____
_____
_____
4.
Water vapor changes to liquid water during which process?
1.
(1) dissolving (3) evaporation
2.
(2) melting (4) condensation
5.
A substance has a freezing point of –38°C and a boiling point of 356°C. At what temperature would this substance be in its liquid state?
1.
(1) –100°C
2.
(2) –50°C
(3) 80°C
(4) 375°C
6.
Water at 20°C in an uncovered pan is evaporating very slowly. What could be done to the water to make it evaporate more quickly?
(1) Cover it.
(2) Heat it.
(3) Place it in the dark.
(4) Put salt in it.
7.
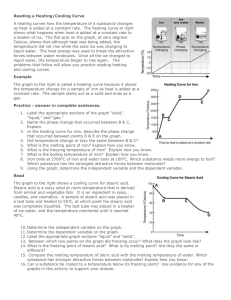
The graph below shows the heating curve for substance X .
At approximately which temperature does a phase change begin?
(1) − 30°C
(2) − 10°C
(3) 0°C
(4) 18°C