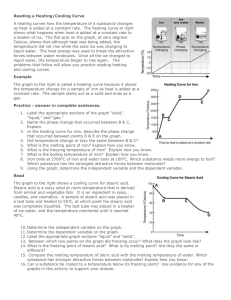
Heating & Cooling of Stearic Acid You will be observing the changes of stearic acid as it changes from solid to liquid by heating, and then from liquid to solid as it cools down again. You will be recording the temperature at a number of points as the ice is heated. Method 1. 2. 3. 4. 5. Take an initial temperature reading before turning on your flame Turn on your flame and begin timing your experiment, making sure to use a small flame Record the temperature on the data results sheet every thirty seconds Stop the experiment once the stearic acid reaches ~70 Degrees On a new table, record the results of the stearic acid cooling again by taking a temperature measurement every thirty seconds 6. Transfer your results to the spreadsheet on Google Classroom Aim: State your aim ______________________________________________________________________________ Prediction: State your prediction ______________________________________________________________________________ Heating Results: Time (mins) Temperature Observations Cooling Results: Time (mins) Temperature Observations