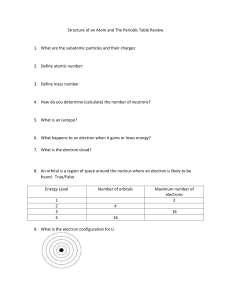
Student Name:__________________________________________________ Homework Assignment #5 1. Identify the group number(s) of the following families on the periodic table. a. Halogens c. Alkali metals e. Alkaline earth metals b. Noble gases d. Transition metals 2. On the following list of elements, circle the symbols of the transition elements and underline the symbols of the halogens: Ca Ag Cl Fe Ni Br Cu F Na 3. Complete the following table by checking the appropriate box for each subatomic particle. Subatomic Particles Charge Positive Negative Neutral Where Found Inside Outside Nucleus Nucleus Proton Electron Neutron 4. Complete the following table of neutral atoms: Element Atomic Number Proton Electron Iron Neutron Mass Number 30 13 19 27 20 Bromine 80 Gold 197 5. Complete the following table of neutral atoms of isotpes: Element 90 Atomic Number Proton Electron Mass Number 22 27 38Sr 20 22 Neutron 10Ne 34 50 82 76 6. Answer the question or complete the following statements about the Periodic Table. a. Group I metals are called b. Elements in the middle of the periodic table c. The sum of the protons and neutrons d. Malleable is a property of a e. The vertical columns are called families or f. The elements are arranged by atomic g. Calcium is a(n) _______ metal. h. A ___ has properties of both metals and nonmetals