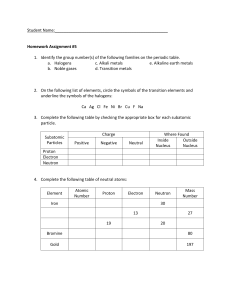
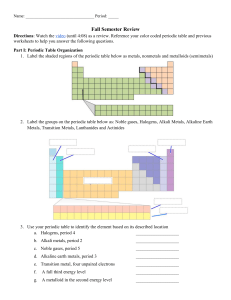
Structure of an Atom and The Periodic Table Review 1. What are the subatomic particles and their charges: 2. Define atomic number: 3. Define mass number 4. How do you determine (calculate) the number of neutrons? 5. What is an isotope? 6. What happens to an electron when it gains or loses energy? 7. What is the electron cloud? 8. An orbital is a region of space around the nucleus where an electron is likely to be found. True/False Energy Level 1 2 3 4 Number of orbitals 4 18 16 9. What is the electron configuration for Li This Photo by Unknown Maximum number of electrons 2 10. What is the electron configuration for C This Photo by Unknown 11. Define energy levels 12. What information can you get from the periodic table 13. What is a period on the periodic table? 14. What is group on the periodic table? 15. What is an atomic mass unit? 16. What information is in each box of the periodic table? 17. The elements within a group have similar properties. True/False 18. Define Metals 19. Define transition metals 20. Define metalloids 21. 22. 23. 24. What group are alkali metals in What group are alkali earth metals in What group are the halogens What group are the noble gases 25. Fill in the chart below: this is an example of how it will be on the test. Make sure you know 1-20! 1 13 S H Al S 1.0079 26.982 32.066 11 Na 22.990