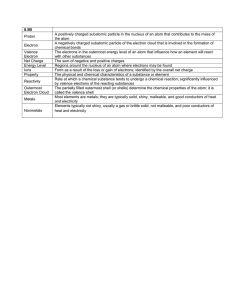
Draw a graph that represents H20 changing states. Label melting
advertisement

Draw a graph that represents H20 changing states. Label melting point, boiling point, solid, liquid, gas. Draw an atom. Label the three subatomic particles that make up the atom. Indicate the charges for each subatomic particle. Using a periodic table how can you determine the number of protons, neutrons and electrons of an element? What are valence electrons? What are they used for? How many protons, neutrons, and electrons? How many valence electrons does Carbon have? (Draw a Bohr model!) What is density? How is it calculated? What is the density for water? If a substance has a density of 2g/ml will it sink of float? How do you know? Define viscosity? Give an example of a liquid that is viscous. Give an example of a liquid that is not viscous. Draw a little periodic table that shows what the periods are. What is matter? What is the difference between physical and chemical properties? Give two examples for each. Draw a little periodic table that shows what the families/groups are. What is an isotope? How does the atomic mass change for an isotope of the same element? How does the atomic number change? Define: element Describe how to find the volume of a cube, a liquid, and an irregular solid. What is mass and how is it measured? Draw a little periodic table. Label the metals, nonmetals, and semimetals. What group is more reactive, noble gases or halogens? What group is more reactive, alkali metals or alkaline earth metals? Define: atom Draw a test tube (a density column) with three liquids. Label the layer that is the most dense. Label the layer that is the least dense. Define: compound What is the difference between a heterogeneous and homogeneous mixture? Give an example of each. Can they both be separated into the original components? Define: mixture Draw a graph that represents the linear relationship between mass and volume. What is the temperature for the melting point of H20? The boiling point? Define: solution Draw a picture that represents the movement of molecules in a gas, liquid and solid. Label the pictures and state which has the most energy and which has the least energy.