COORDINATE COVALENT BONDING WHEN A HYDROGEN ATOM FORMS A COVALENT BOND
advertisement
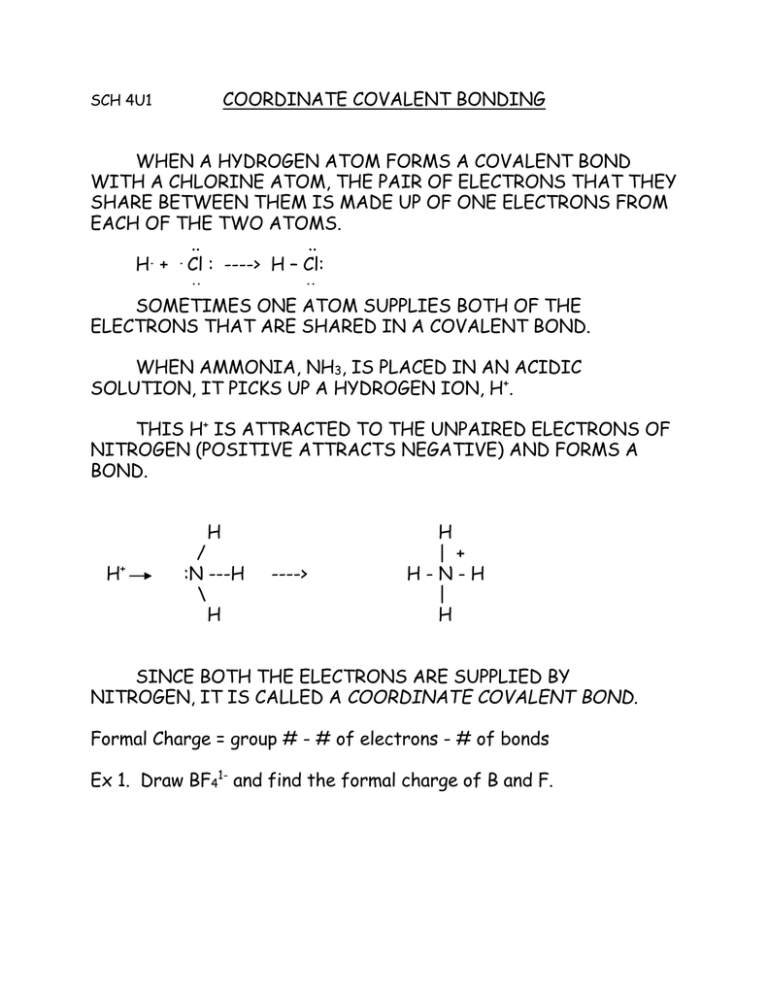
COORDINATE COVALENT BONDING SCH 4U1 WHEN A HYDROGEN ATOM FORMS A COVALENT BOND WITH A CHLORINE ATOM, THE PAIR OF ELECTRONS THAT THEY SHARE BETWEEN THEM IS MADE UP OF ONE ELECTRONS FROM EACH OF THE TWO ATOMS. .. .. . . H + Cl : ----> H – Cl: .. .. SOMETIMES ONE ATOM SUPPLIES BOTH OF THE ELECTRONS THAT ARE SHARED IN A COVALENT BOND. WHEN AMMONIA, NH3, IS PLACED IN AN ACIDIC SOLUTION, IT PICKS UP A HYDROGEN ION, H+. THIS H+ IS ATTRACTED TO THE UNPAIRED ELECTRONS OF NITROGEN (POSITIVE ATTRACTS NEGATIVE) AND FORMS A BOND. H H + / :N ---H \ H ----> H | + H-N-H | H SINCE BOTH THE ELECTRONS ARE SUPPLIED BY NITROGEN, IT IS CALLED A COORDINATE COVALENT BOND. Formal Charge = group # - # of electrons - # of bonds Ex 1. Draw BF41- and find the formal charge of B and F.