Presentation10_108
advertisement

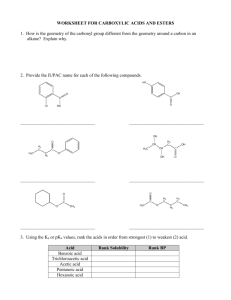
By: Dr. Shatha Alaqeel 1 1) Aldehydes General formula: RCHO or RCH=O The aldehyde group is always at the end of a chain, so it will always take number 1. 2 Common nomenclature The common names of aldehydes are derived from the corresponding acids: O O H OH Formic acid H3C H OH Acetic acid H3C H Formaldehyde H3C OH Propionic acid O O O O H3C OH Butyric acid O O H Acetaldehyde H3C H Propionaldehyde H3C H Butyraldehyde 3 IUPAC Nomenclature Select the longest continuous carbon chain that contains the C=O group and replace the ending by the suffix al The CHO group is assigned the number 1 position and takes precedence over other functional groups that may the present such as –OH, C=C for example. 4 O H C H H3C IUPAC Methanal Cl H3C O O H3C CH2 H Ethanal O CH 2-Chloropropanal C H H3CH2CH2C Propanal HO H O O C C Butanal O H 3-Hydroxypropanal H3CHC=HC C H 2-Butenal 5 H Aromatic aldehydes are usually designated as derivatives of the simplest aromatic aldehyde, Benzaldehyde. O O H H O2N Benzaldehyde OH p-Nitrobenzaldehyde O O H H MeO o-Hydroxybenzaldehyde (Salicyladehyde) p-Methoxybenzaldehyde (Anisaldehyde) 6 2) Ketones General formula: RCOR’ (R and R’=alkyl or aryl) Common name: listing the alkyl substitutents attached to the carbonyl group, followed by the word ketone. IUPAC system: relpace the ending –e by the suffix –one. The chain is numbred in such a way as give the lowest number to the C=O group. O H3C C O CH3 Common Dimethyl ketone Acetone IUPAC Propanone H3C C O C6 H 5 Methyl phenyl ketone H3C Methyl vinyl ketone Acetophenone Phenyl ethanone C O CH=CH 2 H5C6 C C6H5 Diphenyl ketone Benzophenone 3-Buten-2-one Diphenylmethanone 7 O O C2 H 5 O OH CHO C C2 H 5 Cyclopentylpropanone 3-Ethyl-2-hydroxycyclohexanone 5-Oxohexanal 8 PHYSICAL PROPERTIES OF KETONES AND ALDEHYDE O C + O C O C - C O Because the polarity of the carbonyl group, aldehydes and ketones are polar compounds. Dipole-dipole attractions, although important, are not as strong as intractions due to hydrogen bonding. As a result, the boiling points of aldehydes and ketones are higher than those of nonpolar alkanes, but lower than those of alcohols. C O H O H O C The lower aldehydes and ketones are soluble. 9 Preparation of aldehydes and ketones 1- Oxidation of alcohols RCH 2 OH CrO 3/ pyridine O R Cu / heat H O CrO 3/ pyridine R2CH R OH C R Cu / heat 2- Ozonolysis of alkenes A A A A 1)O 3 2)Zn / H 2O A O A + A O A 10 3- Hydration of alkynes H C C + HO H H2SO4, HgSO4 H C C OH an enol unstable C C H O carbonyl more stable 4- Friedel-Craft’s acylation O O + AlCl 3 R Cl CH3 11 REACTIONS OF ALDEHYDES AND KETONES 1- Reduction of carbonyl group 2 H 2 / Pd H3C OH H3C O H 1) NaBH 4 2) H 2O 12 H3C OH 2- Addition of Grignard Reagents: Formation of alcohols R R' O R + C R'MgX H 1) Dry ether R HO + C OH 2) H 2O O H3C CH C2H5MgX H 1) Dry ether 2) H 2O H3C CH C2H5 R' O C R' + R''MgX 1) Dry ether 2) H 2O R C OH R'' CH3 O + CH 3MgX 13 1) Dry ether 2) H 2O OH 3- Oxidation reaction aR-CHO or Ar-CHO KMnO4 or RCOOH ArCOOH or K Cr O 2 2 7 b- Iodoform reaction: The reaction occurs in any aldehyde or ketone containing CH3CO. O H3C C O + 3 I2 + 4 NaOH R O Na R CH3 H3C I2 / NaOH - H3C COONa + + CHI3 + + 3 NaI CHI3 O 14 4- Addition of Hydrogen Cyanide: Formation of cynohydrins R' O R C + R' R HCN C OH CN Cyanohydrin CN O H NH2 OH + OH H2 / Pt + or LiAlH 4 and H 3O HCN Benzaldehyde cyanohydrin O OH + CN HCN H3O OH + COOH Heat 5- Addition of acetylide ions: R' O R C R' + - 2 C Na R C H3 O + + R C C C R OH O + H3C 15 C - C Na + H3O + OH C C CH3 2 6- Addition of alcohols: R'O O C R R'O + + 2 R'OH H R''OH C R OH 2 R Hemiacetal 2 Ketone Hemiketal R =H: R =Alkyl O H3C + H3C H H 5C 2O CH OC 2H5 H3C C H3C CH3 C OC 2H5 CH3 Hemiketal 16 + H H3C CH OC 2H5 Acetal H5C2O H C2H5OH C2H5OH HO + + 2 Ketal Hemiacetal O OR'' R Acetal H C2H5OH C HO + C H 2 R Aldehyde R + C2H5OH + H H3C C CH3 Ketal OC 2H5 7- Addition of Ammonia and Ammonia Derivatives NH3 C NH Imine NH 2OH Hydroxylamine C O H2N NH2 Hydrazine 17 C N OH Oxime C N NH2 Hydrazone