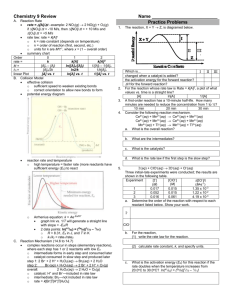
H 3 AsO 4 + 3 I - + 2 H 3 O + ® H 3 AsO 3 + I 3
advertisement

Chemistry 9: Kinetics A. Reaction Rate (14.1 to 14.4) 1. rate = [A]/t a. A is a general term for reactant or product b. usually an absolute value, but not always c. instantaneous rate = slope of [A] vs. t graph d. units depend on units for [ ] and t, but usually M/s e. same reaction has different rates 2 NO2(g) 2 NO(g) + O2(g) rate = [NO2]/t = -[NO]/t = -2[O2]/t 2. rate law: rate = k[A]n a. k = rate constant 1. depends on nature of reaction (larger k means faster reaction) 2. depends on temperature (k increases with temperature) b. n = order of reaction (first, second, etc.) c. multiple reactants: rate = k[A]m[B]n (overall order = m + n) d. determined experimentally: process write rate laws for each experiment divide rate laws to cancel out all but one reaction order solve for unknown reaction order repeat solve for k e. units for k are Mxt-1, where x = (1 – overall order) 3. summary chart Order 0 1 2 k k[A] k[A]2 rate = kt = [A]o – [A]t ln([A]o/[A]t) 1/[A]t – 1/[A]o t½ = [A]o/2k ln2/k 1/k[A]o [A] vs. t ln[A] vs. t 1/[A] vs. t linear Plot B. Collision Model (14.5) 1. effective collision a. sufficient speed to weaken existing bonds b. correct orientation to allow new bonds to form 2. 3. reaction process a. effective collision between reactants b. activated complex: partial bonding (unstable) c. spontaneous formation of products potential energy diagram a. reactant, activated complex and product energies b. activation energy (Ea) 1. Ea = Eactivated complex – Ereactants 2. positive quantity, which depends on nature of reactants, but not temperature or [ ] 3. H = Ea - Ea' (Ea’ for reverse reaction) Name __________________________ 4. reaction rate and temperature a. high temperature = faster rate 1. more particles' kinetic energy > Ea 2. more frequent collisions Arrhenius equation: k = Ae-Ea/RT 1. graph lnk vs. 1/T will generate a straight line with slope = -Ea/R 2. calculating Ea: Ea = -R(slope) 3. 2 data points: ln(k1/k2) = (Ea/R)(1/T2 – 1/T1) a. R = 8.31, Ea in J, and T in K b. k1/k2 = rate1/rate2 Reaction Mechanism (14.6 to 14.7) 1. complex reactions occur in steps (elementary reactions), where each step has 1 or 2 reactants with low Ea b. C. 2. 3. 4. Step 1: NO2(g) + NO2(g) NO3(g) + NO(g) Step 2: NO3(g) + CO(g) NO2(g) + CO2(g) Overall: NO2(g) + CO(g) NO(g) + CO2(g) intermediate forms in early step and is consumed in a subsequent step (NO3) corresponds exactly to rate law a. coefficients of slow step (rate-determining step) become exponent in rate law b. example: if step 1 is slow step, then rate = k[NO2]2 catalyst a. catalyst provides a reaction mechanism that has a lower Ea than the noncatalyzed reaction b. c. homogeneous catalyst 1. consumed in slow step, reappear in fast step 2. example: decomposition of H2O2 step 1: 2 Br- + 2 H+ + H2O2(aq) Br2(aq) + 2 H2O step 2: Br2(aq) + H2O2(aq) 2 Br- + 2 H+ + O2(g) overall: 2 H2O2(aq) 2 H2O + O2(g) a. catalyst: H+ and Br-—included in rate law b. intermediate: Br2—not included c. rate = k[Br-]2[H+]2[H2O2] heterogeneous catalyst (different phase from reactants)—usually solid (written over arrow) Experiments 1. Reaction Rate Lab—Measure the time it takes to consume a reactant while varying the concentrations and conditions of the experiment and use the data to develop a rate law and determine the activation energy. This experiment involves the reaction: 6 I-(aq) + BrO3-(aq) + 6 H+(aq) 3 I2(aq) + Br-(aq) + 3 H2O, which is reasonable slow. a. What is the rate law for this reaction? In order to determine the rate law, we measure the time it takes a fixed amount of I2 to form under varied conditions. To fix the amount of I2 that is produced, we add a second reaction: I2(aq) + 2 S2O32-(aq) 2 I-(aq) + S4O62-(aq), which is essentially instantaneous. Thus, the I2 produced in the first reaction is consumed immediately in the second reaction until all the thiosulfate, S2O32-, is consumed. After which, the I2 turns the solution blue as it reacts with the starch indicator. Reaction rate, rate = [S2O32-]/t, where [S2O32-] = 0.00020 M and t = time from when KBrO3 is added until a noticeable blue color appears. b. What is the purpose of thiosulfate, S2O32-? c. What is the purpose of the starch? Ea is determined by calculating k at different temperatures, graphing lnk vs. 1/T, and then finding the slope of the line. d. How is Ea determined? Experiment 1: Clean three small test tubes. Add 10 drops of 0.010 M KI, 10 drops 0.001 M Na2S2O3, 10 drops of 0.10 M HCl, 1 drops of starch indicator and 10 drops of distilled water to each test tube. Mix the contents by shaking the test tubes. Wait 2 minutes to be sure that no reaction occurs (turns blue). Add 10 drops of 0.040 M KBrO3 to each test tube and mix. Start timing as soon as the first drop of KBrO3 touches the contents of the test tube and stop timing when you first notice a blue color. Record the time (in seconds) in the table for number 1. Measure the temperatures of the contents of the test tubes and record the temperature in the table. Experiments 2-5: Repeat the above procedures using the drops listed for experiments 2-5. Experiment 6-7: Repeat the above procedures except place the test tubes in a 250-mL beaker of water at 5oC (experiment 6) or 50oC (experiment 7) for five minutes before adding the KBrO3. Return the test tubes to the beaker as soon as the KBrO3 is added until the color change first begins. Record the time and temperature. Experiment 8: Repeat the above procedures except replace 1 of the drops of water with 1 drop of (NH 4)2MoO4. e. Record the time it takes for the mixture to turn blue. Reaction Mixtures (volumes in drops) Time (s) Number Mixture Temp Na2S2O3 HCl H2 O Starch KBrO3 KI Trial 1 Trial 2 Trial 3 Average 1 Control 10 10 10 10 1 10 2 2 x [KI] 20 10 10 0 1 10 3 2 x [HCl] 10 10 20 0 1 10 4 2 x [KBrO3] 10 10 10 0 1 20 5 Verification 8 10 8 12 1 12 6 Cold 10 10 10 10 1 10 7 Hot 10 10 10 10 1 10 8 Catalyst 10 10 10 9 1 10 Calculate the rate for each reaction mixture 1 2 3 4 rate = 0.00020/tav g. Calculate the order for each reactant by completing the chart. [H+]n [I-]m rate 3 ( ) rate 2 ( ) = = 2n n= = = 2m m = rate 1 rate 1 ( ) ( ) f. 5 6 7 [BrO3-]P ) rate 4 ( = = 2p p= rate 1 ( ) h. Calculate the rate constant (k) for each reaction based on the rate law: k = rate/[I-][H+]2[BrO3-], include units. 1 2 3 4 Average ( ) ( ) ( ) ( ) k= k= k= k= (0.002)(0.02)2(0.008) (0.004)(0.02)2(0.008) (0.002)(0.04)2(0.008) (0.002)(0.02)2(0.016) k= k= k= k= i. Compare the predicted rate for reaction 5 based on the rate law with the actual rate from experiment 5 above. [H+] = (8/50)[0.10] [BrO3-] = (12/50)[0.040] rate data % difference [I-] = (8/50)[0.010] rate = k[I-][H+]2[BrO3-] j. Calculate the rate constant (k = rate/[I-][H+]2[BrO3-]), lnk, temperature in Kelvin (TK), and 1/TK for reaction 6, 1 and 7. k lnk TK 1/TK Number Temperature 6 1 7 kj. (1) Determine a scale for the y-axis (lnk) so that the values in the chart above span the whole graph. Graph lnk vs 1/TK. Draw a best fit straight line (with a ruler!). lnk (2) Determine k at 30oC from the graph. (3) Calculate the time it should take reaction mixture 1 to turn blue at 30oC. (4) Determine the slope of the line from the graph. (5) Calculate Ea = (8.31 J/mol•K) x |Slope|. 0.0030 0.0032 0.0034 1/T (K-1) 0.0036 Practice Problems 1. A. Reaction Rate Consider the graph of [ ] vs. t for the reaction. 2 NO2(g) 2 NO(g) + O2(g) 2. c. Calculate the reaction rate of NO at 100 s. d. Calculate the reaction rate of O2 at 100 s. The following data were measured for the reaction below: 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g) [NO] (M) [H2] (M) Initial Rate (M/s) Experiment 1 0.10 0.10 1.2 x 10-3 2 0.10 0.20 2.4 x 10-3 3 0.20 0.10 4.8 x 10-3 a. Using these data, determine the order of the reaction (1) with respect to NO (2) with respect to H2 a. b. Calculate the average rate of NO2 between 50 s and 150 s. Calculate the reaction rate of NO2 at 100 s. b. Write the rate law for the reaction. c. Calculate the rate constant (include units). d. Calculate the rate when [NO] = 0.050 M and [H2] = 0.150 M? 3. The initial rate of reaction for: X(g) + 2Y(g) 3 Z(g) was measured at different concentrations with the results: [X] (M) 0.10 0.20 0.20 [Y] (M) 0.10 0.10 0.20 Rate (M/s) 0.30 0.60 2.4 a. Calculate the reaction orders for X Y 4. b. What is the overall order for the reaction? c. Write the rate law for the reaction. d. Calculate k for the reaction. e. Calculate the rate when [X] = 0.1 M and [Y] = 0.2 M. SO2Cl2(g) SO2(g) + Cl2(g) The reaction is first order and the k = 2.2 x 10-5 s-1. a. Calculate the time to react 20.% of the SO2Cl2? b. The initial concentration of SO2Cl2 = 0.16 M, how much is left after six hours? 9. 2 NO(g) + Cl2(g) 2 NOCl(g) The activation energy is 17.5 kJ/mol. At 0oC the rate constant is 4.50 M-2s-1. At what temperature will the rate constant be 8.00 M-2s-1? 10. 2 NO2(g) 2 NO(g) + O2(g) At 319oC its rate constant is 0.498 M-1s-1 and at 383oC its rate constant is 4.74 M-1s-1. Calculate Ea. C. Reaction Mechanism 11. Consider the following mechanism: step 1: NO(g) + Br2(g) NOBr2(g) slow step 2: NOBr2(g) + NO(g) 2 NOBr(g) fast a. Write the overall equation for the reaction. b. Write the rate law for this reaction. 12. Consider the following mechanism. step 1: CH4(g) + Cl(g) CH3(g) + HCl(g) step 2: CH3(g) + Cl2(g) CH3Cl(g) + Cl(g) a. Write the overall equation of the reaction. b. Identify the following. catalyst intermediate slow step c. 5. 6. What is the half-life of this reaction? C6H12O3(g) 3 C2H4O(g) The reaction is first order and k = 3.0 x 10-4 s-1. If we start with 0.25 M C6H12O3, what will its concentration be after 10. min? The decomposition of HI, 2 HI(g) H2(g) + I2(g), is a second order reaction with a rate constant of 2.8 x 10 -4 s-1. a. If [HI]o = 1.0 M, how long will it take for the concentration to decrease by 25 %? rate law 13. Consider the following mechanism. step 1: NO2(g) + SO2(g) NO(g) + SO3(g) step 2: NO(g) + ½ O2(g) NO2(g) a. Write the overall equation of the reaction. b. Identify the following. catalyst intermediate slow step rate law c. b. 7. How do you know that this a homogeneous catalyst? If [HI]o = 1.0 M, what [HI] after 2.0 hours? B. Collision Model Label Ea, Ea', and H on the following potential energy diagrams. Indicate which diagram represents an exothermic reaction and which represents and endothermic reaction 14. Hydrogen peroxide decomposes to water and oxygen according to the following reaction. 2 H2O2(aq) 2 H2O(l) + O2(g) a. 0.100 mol/L of H2O2 is consumed in 72.0 min. (1) What is the average rate of H2O2 consumption? (2) What is the average rate of O2 formation? Initial rate determination at 40oC for the decomposition given the following data: [H2O2] (mol/L) 0.100 0.200 0.300 Rate (mol/L•min) 1.93 x 10-4 3.86 x 10-4 5.79 x 10-4 (1) What is the order of the reaction? b. 8. Use the diagram in your notes (B4) to explain why a reaction occurs at a faster rate at higher temperature. (2) Write the rate law for the reaction. What is the experimental rate law? (A) Rate = k[NO][O2] (B) Rate = k[NO][O2]2 2 (C) Rate = k[NO] [O2] (D) Rate = k[NO]2[O2]2 (3) Calculate the rate constant k. c. If a 30.0 % solution is kept at 40oC, how long will it take for it to become 10.0 % H2O2? d. It has been determined that at 50oC, the rate constant is 4.32 x 10-3 min-1. Calculate the activation energy. Questions 5-6 The oxidation of iodide ions by arsenic acid in acidic aqueous solution occurs according to the reaction. H3AsO4 + 3 I- + 2 H3O+ H3AsO3 + I3- + H2O The rate law is: Rate = k[H3AsO4][I-][H3O+] 5. What is the order of the reaction with respect to I-? (A) 0 (B) 1 (C) 2 (D) 3 6. e. (1) Determine k at 4oC. (2) Determine how long it would take for a 30.0 % solution to decompose to 10.0 % at 4oC? f. The rate constants for the uncatalyzed and catalyzed reactions at 25oC are 5.21 x 10-4 min-1 and 2.95 x 108 min-1 respectively. Calculate the half-life for the (1) uncatalyzed reaction? According to the rate law for the reaction, an increase in the concentration of H3O+ has what effect on this reaction? (A) The rate of reaction increases. (B) The rate of reaction decreases. (C) The value of the rate constant increases. (D) The value of the rate constant decreases. Questions 7-8 The concentrations of X measured over a period of time for the reaction X + Y Z is graphed below. (2) catalyzed reaction? g. Hydrogen peroxide in basic solution oxidizes iodide ions to iodine. The proposed mechanism is H2O2(aq) + I-(aq) HOI(aq) + OH-(aq) slow HOI(aq) + I-(aq) I2(aq) + OH-(aq) fast (1) Write the overall redox reaction. (2) Write a rate law consistent with this proposed mechanism. Practice Multiple Choice Briefly explain why the answer is correct in the space provided. Questions 1-2 refer to the following types of energy. (A) Activation energy (B) Free energy (C) Ionization energy (D) Lattice energy 1. The energy in a chemical or physical change that is available to do useful work 2. The energy required to form the transition state in a chemical reaction 3. For the reaction whose rate law is, Rate = k[X], a plot of which of the following vs. time is a straight line? (A) [X] (B) 1/[X] (C) ln[X] (D) [X]2 4. The initial-rate data in the table were obtained for the reaction: 2 NO(g) + O2(g) 2 NO2(g). [NO]o [O2]o Initial Rate of Formation Exp (mol•L-1) (mol•L-1) of NO2 (mol•L-1•s-1) 1 0.10 0.10 2.5 2 0.20 0.10 5.0 3 0.20 0.40 80. 7. What is the half-life for this reaction? (A) 2 minutes (B) 7 minutes (C) 10 minutes (D) half-life is not constant 8. What is the order of reaction with respect to X? (A) 0 (B) 1 (C) 2 (D) can't be determined 9. How long does it take in minutes for the partial pressure of the reactant in a first order reaction to decrease from 1.0 atm to 0.125 atm, where the half-life is 19 minutes? (A) 38 (B) 57 (C) 76 (D) 152 10. Which is associated with relatively slow reaction rates? (A) The presence of a catalyst (B) High temperature (C) High concentration of reactants (D) Strong bonds in reactant molecules 11. Which of the following best describes the role of a match in setting a fire? (A) The match decreases Ea for the slow step. (B) The match increases the concentration of the reactant. (C) The match supplies Ea for the combustion reaction. (D) The match provides a more favorable activated complex for the combustion reaction. 12. 2 A(g) + B(g) 2 C(g) Which best explains the observation that there is no change in reaction rate when the concentration of B(g) is doubled? (A) The order of the reaction with respect to B is 1. (B) The overall order of the reaction is 0. (C) B is not involved in the rate-determined step. (D) substance B is a catalyst. 13. 2 NO + O2 2 NO2 The mechanism for the overall reaction is the following. (1) NO + NO N2O2 slow (2) N2O2 + O2 2 NO2 fast Which rate law is consistent with this mechanism? (A) Rate = k[NO]2 (B) Rate = k[NO][O2]-1 (C) Rate = k[NO]2[O2]-1 (D) Rate = k[NO]2[O2] 14. A reaction was observed for 20 days and the percentage of the reactant remaining after each day was recorded below. time 0 1 2 3 4 5 6 7 10 20 % 100 79 63 50 41 31 25 20 10 1 Which best describes the order and half-life of the reaction? (A) First order with a 3 day half-life (B) First order with a 10 day half-life (C) Second order with a 3 day half-life (D) Second order with a 6 day half-life Questions 15-17 refer to the proposed steps for the catalyzed reaction between Ce4+ and Tl+. Step 1: Ce4+ + Mn2+ Ce3+ + Mn3+ Step 2: Ce4+ + Mn3+ Ce3+ + Mn4+ Step 3: Mn4+ + TI+ Tl3+ + Mn2+ 15. The products of the overall catalyzed reaction are (A) Ce4+ and TI+ (B) Ce3+ and Tl3+ (C) Ce3+ and Mn3+ (D) Ce3+ and Mn4+ 16. The catalyst for the reaction is (A) Ce4+ (B) Mn2+ (C) Ce3+ 19. Which of the following is true for this reaction? (A) The reaction is exothermic where Ea > |H| (B) The reaction is endothermic where Ea > |H|. (C) The reaction is exothermic where Ea < |H|. (D) The reaction is endothermic where Ea < |H|. 20. The addition of a catalyst to this reaction would cause a change in which of the indicated energy differences? (A) I only (B) II only (C) Ill only (D) I and II only Practice Free Response 1. Consider the reaction: 2 HI(g) H2(g) + I2(g), where the rate of the reaction in terms of [HI] = -0.100 M•min-1. a. What is the rate of the reaction in terms of H2(g)? b. 2. What is the rate of the reaction in terms of I2(g)? Consider the reaction: A + B2 Products. The following experimental data at 22oC were obtained: B2 (M) Rate (M•s-1) A (M) 0.100 0.100 0.080 0.500 0.100 0.40 0.100 0.500 2.0 a. What is the order of the reaction with respect to each reactant? b. What is the rate constant for the reaction, including units? c. What would cause an increase in the rate constant? d. The activation energy for the reaction is 115 kJ/mol. What is the rate constant of the reaction at 27oC? (D) Mn4+ 17. Intermediates in the reaction are (A) Ce4+ and Ce3+ (B) Ce3+ and Mn3+ + 3+ (C) Tl and Tl (D) Mn3+ and Mn4+ 18. Questions 19-20 The energy diagram for the reaction X + Y Z is shown below. Rate = k[M][N]2 If the concentrations of M and N are doubled, the reaction rate will increase by a factor of (A) 2 (B) 4 (C) 6 (D) 8 3. 4. 5. Consider the first-order decomposition of A. The rate constant is 1.7 x 10-2 min-1. a. Calculate the rate of the reaction when the initial concentration of A is 0.400 M. b. What percent of A will be used up in two hours? c. What is the half-life of the reaction? 6. An environmental concern is the depletion of O3 in Earth's upper atmosphere, where O3 is normally in equilibrium with O2 and 0. A proposed mechanism for the depletion of O3 in the upper atmosphere is shown below. Step I O3 + CI O2 + CIO Step II CIO + O CI + O2 a. Write a balanced equation for the overall reaction represented by Step I and Step II above. b. Clearly identify the catalyst in the mechanism above. Justify your answer. The redox reaction between Tl+ by Ce4+ occurs by the following mechanism. Ce4+(aq) + Mn2+(aq) Ce3+(aq) + Mn3+(aq) Ce4+(aq) + Mn3+(aq) Ce3+(aq) + Mn4+(aq) Mn4+(aq) + Tl+(aq) Mn2+(aq) + Tl3+(aq) a. What is the balanced equation for the overall reaction? c. Clearly identify the intermediate in the mechanism above. Justify your answer. b. What molecule acts as a catalyst for this reaction? d. c. What molecules is an intermediate for this reaction? If the rate law for the overall reaction is found to be rate = k[O3][CI], determine the following. (1) The overall order of the reaction d. The rate law for this reaction is rate = k[Ce4+][Mn2+]. Which step is the slow step in the mechanism? I-(aq) + CIO-(aq) IO-(aq) + Cl-(aq) Three initial-rate experiments were conducted; the results are shown in the following table. Experiment [ClO-] [I-] [IO-]/t (M) (M) (M•s-1) 1 0.017 0.015 0.156 2 0.052 0.015 0.476 3 0.016 0.061 0.596 a. Determine the order of the reaction with respect to each reactant listed below. Show your work. (1) I- (2) CIO- b. For the reaction, (1) write the rate law for the reaction. (2) calculate rate constant, k, and specify units. c. The rate constant for this reaction is determined at various temperatures. The data is graphed in order to determine the activation energy, Ea. (1) What variables are graphed? (2) Explain how to calculate the activation energy from this graph. (2) Appropriate units for the rate constant, k (3) The rate-determining step of the reaction, along with justification for your answer