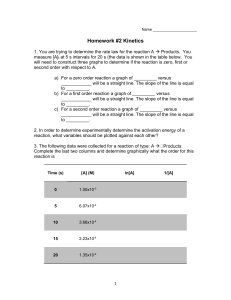
Kinetics 2

NAME _______________________________________________ __________/4
RULES:
For any credit you must answer every question, in the event of multiple questions. No part(s) may be skipped.
You should print out this page, and show your work and your answer(s) on this page. Pencil/Pen/Type are each acceptable.
You must turn in this page, by the end of the school day (2:10 p.m) on which it is due.
Partial credit is assigned only under particular circumstances, upon which I decide. (e.g. double jeopardy)
The earned points will be applied to your overall point score, as bonus.
The work must be absolutely legible to me.
If I must guess as to meaning, I shall always guess, incorrectly.
You are welcome to use your text, Trivedi flash, internet sources, a friend’s understandings for research purposes. All work however, must be your own. Extended responses must reflect your understanding of the issue …hence, NO group work & you must work in good faith.
Every question is worth 0.5 points.
AP BONUS 2: JUST A KINETICS REVIEW ….TO KEEP IT FRESH
DUE: 27 OCTOBER 2015
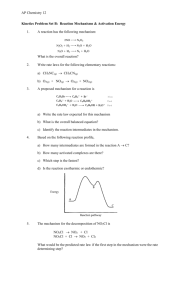
1) A reaction occurs by the following mechanism. step 1: Ce (4+) + Mn (2+) → Ce (3+) + Mn (3+) step 2: Ce (4+) + Mn (3+) → Ce (3+) + Mn (4+) step 3: Tl
(+)
+ Mn
(4+)
→ Ti
(3+)
+ Mn
(2+)
Create a reaction coordinate diagram (a potential energy diagram) for the reaction mechanism shown with the following guidelines. Be sure that each of the E a
values the overall E a
value and the ∆H are labeled.
The Ea for step 1 is 50 kJ
The Ea for step 2 is 50 kJ
The Ea for step 3 is 100 kJ
The overall Ea for the whole reaction is 150 kJ
The reaction has a ∆H = +50 kJ/mol
PE (kJ) reaction coordinate turn this over and go on
2) The mechanism for the decomposition of NO
2
Cl is
NO
2
Cl
NO
2
+ Cl slow
NO
2
Cl + Cl
NO
2
+ Cl
2
fast a) What is the overall balanced reaction equation reaction? _________________________________ b) What would be the predicted rate law? ___________________________
_____3) A multi-step reaction includes an intermediate and a catalyst. The reactions are as follows:
A + X →Y
Y +B → Z
Z + A → C + X
Based on the reactions, which of the following is/are true?
I) The overall balanced equation is A + B → C
II) X is a catalyst
III) Y is an intermediate
1) I, II and III are each accurate
2) Only I and II are accurate
3) Only II is accurate
4) Only II and III are accurate
5) Only III is accurate