CH 117 Fall 2015Worksheet 4 For the following species, which
advertisement

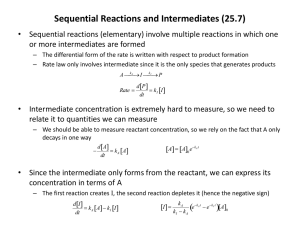
CH 117 Fall 2015 Worksheet 4 1. For the following species, which species is acting as the catalyst? The intermediate? Step 1: Cl + O3 —> ClO + O2 Step 2: ClO + O —> Cl + O2 For this reaction ClO is the intermediate as it is found in the product of the first step and the reactant of the second step. Cl is the catalyst of this reaction as it is seen in the reactant of the first step and the product of the second step. 2. The following mechanism is proposed: 2 NO2 NO3 + NO (slow) NO3 + CO NO2 + CO2 (fast) What are the intermediates involved? What is the overall reaction? What is the rate law for each individual step? What is the rate law for the overall reaction? NO3 is an intermediate in this reaction – intermediates are produced/consumed in one step and consumed/produced in the subsequent steps the species you can cross out when you add all the steps of a mechanism together. To find the overall reaction, add the steps of the mechanism: NO2 + CO NO + CO2 Since all steps of a mechanism are assumed to be elementary, we can read their rate laws and orders straight off of the balanced equation. For the first step: rate = k[NO2]2 Second step: rate = k[NO3][CO] The rate law for the reaction is determined by the slow step – so every step before the slow step is important and those steps that come after the slow step are unimportant. In this case, the first step is the slow step. Whenever this is the case, the rate law for the overall reaction matches the rate law for the slow step. The rate law for the slow step (since it is an elementary step) can be read straight off of the equation. So for the overall reaction, rate = k[NO2]2. 3. The following mechanism is proposed: O3 O2 + O (fast, in equilibrium) O3 + O 2 O2 (slow) What are the intermediates involved in this reaction? What is the overall reaction? What is the rate law for each individual step? What is the rate law for the overall reaction? The intermediate in this reaction is O. The overall reaction is 2 O3 3 O2. First step rate law (since it is a fast step in equilibrium, we need to write both the forward and the reverse rate laws): rateforward = kf[O3]; ratereverse = kr[O2][O] Second step rate law: rate = k[O3][O] Writing the rate law for the overall reaction is a little more complicated in this case. This is because the slow step is last in the mechanism, which means all the steps CH 117 Fall 2015 Worksheet 4 before it do matter and contribute to our rate law. The first step in the proof is to write the rate law for both of the elementary steps, which we did above. Since the first reaction is in equilibrium, the rate of the forward reaction is equal to the rate of the reverse reaction kf[O3] = kr[O2][O]. The rate law for the second step is a little less complicated rate = k[O3][O]. The problem with this rate law is that it includes an intermediate (O). We must replace this [O] in the second step rate law with something else in order to determine the overall rate law – the overall rate law CANNOT contain any intermediates. From our equilibrium rate law, we can solve for [O] and get something to replace it with. In this case, [O] = kf[O3]/kr[O2] since the k’s are both constants, we can combine them and write this expression like this: [O] = k[O3]/[O2]. Now we can replace the [O] in our rate law equation with this expression. Rate = k[O3][O] becomes rate = k[O3] * k[O3]/[O2] again we can combine the k’s to get our final, overall rate law: rate = k[O3]2/[O2]. 4. Choose all of the following that affects the reaction rate as each of the components increase and explain why a) temperature - as the temperature of the reaction increases, the reaction rate increases as it causes greater collisions between molecules. b) concentration – as the concentration of the reactant increase, the reaction rate increases as there are more reactants in the reaction, allowing for the reaction rate to increase. c) surface area- the larger the surface area, the faster the reaction takes place d) catalyst – catalyst decreases the activation energy so that there will be a faster rate of reaction. e) state of subdivision of solid reactants