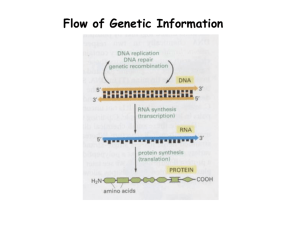
AP Chemistry
advertisement
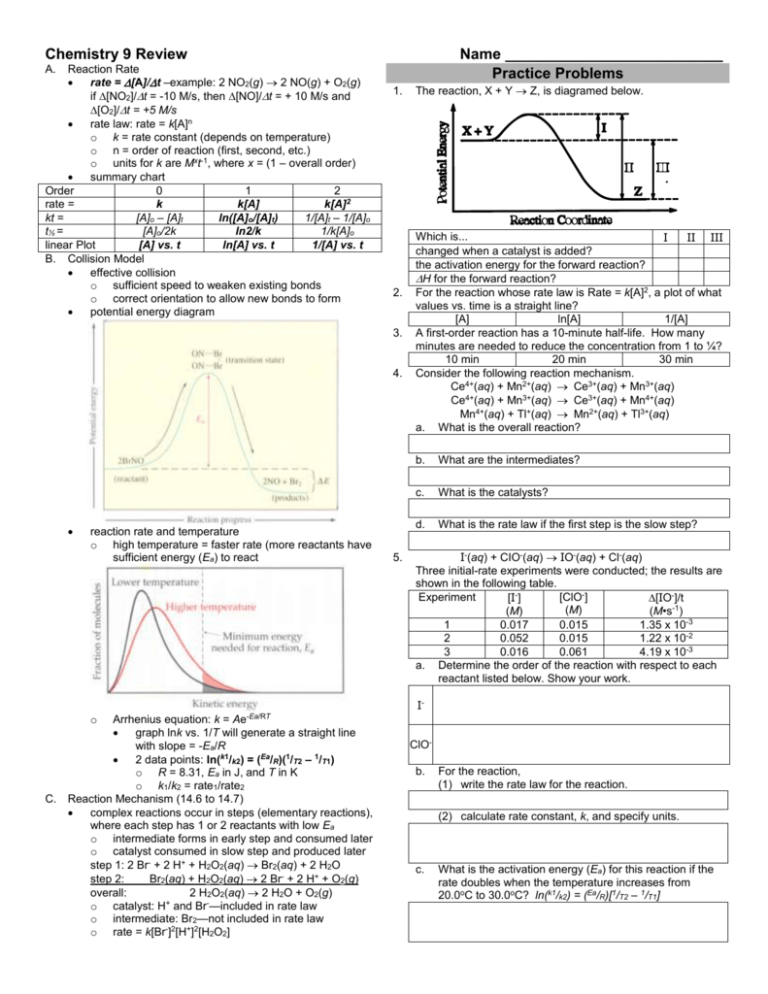
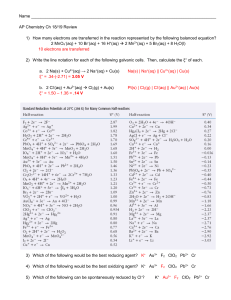
Chemistry 9 Review Reaction Rate rate = [A]/t –example: 2 NO2(g) 2 NO(g) + O2(g) if [NO2]/t = -10 M/s, then [NO]/t = + 10 M/s and [O2]/t = +5 M/s rate law: rate = k[A]n o k = rate constant (depends on temperature) o n = order of reaction (first, second, etc.) o units for k are Mxt-1, where x = (1 – overall order) summary chart Order 0 1 2 k k[A] k[A]2 rate = kt = [A]o – [A]t ln([A]o/[A]t) 1/[A]t – 1/[A]o t½ = [A]o/2k ln2/k 1/k[A]o [A] vs. t ln[A] vs. t 1/[A] vs. t linear Plot B. Collision Model effective collision o sufficient speed to weaken existing bonds o correct orientation to allow new bonds to form potential energy diagram Name __________________________ Practice Problems A. 1. 2. 3. 4. reaction rate and temperature o high temperature = faster rate (more reactants have sufficient energy (Ea) to react 5. The reaction, X + Y Z, is diagramed below. Which is... I II III changed when a catalyst is added? the activation energy for the forward reaction? H for the forward reaction? For the reaction whose rate law is Rate = k[A]2, a plot of what values vs. time is a straight line? [A] ln[A] 1/[A] A first-order reaction has a 10-minute half-life. How many minutes are needed to reduce the concentration from 1 to ¼? 10 min 20 min 30 min Consider the following reaction mechanism. Ce4+(aq) + Mn2+(aq) Ce3+(aq) + Mn3+(aq) Ce4+(aq) + Mn3+(aq) Ce3+(aq) + Mn4+(aq) Mn4+(aq) + Tl+(aq) Mn2+(aq) + Tl3+(aq) a. What is the overall reaction? b. What are the intermediates? c. What is the catalysts? d. What is the rate law if the first step is the slow step? I-(aq) + CIO-(aq) IO-(aq) + Cl-(aq) Three initial-rate experiments were conducted; the results are shown in the following table. Experiment [ClO-] [I-] [IO-]/t (M) (M) (M•s-1) 1 0.017 0.015 1.35 x 10-3 2 0.052 0.015 1.22 x 10-2 3 0.016 0.061 4.19 x 10-3 a. Determine the order of the reaction with respect to each reactant listed below. Show your work. I- o C. Ae-Ea/RT Arrhenius equation: k = graph lnk vs. 1/T will generate a straight line with slope = -Ea/R 2 data points: ln(k1/k2) = (Ea/R)(1/T2 – 1/T1) o R = 8.31, Ea in J, and T in K o k1/k2 = rate1/rate2 Reaction Mechanism (14.6 to 14.7) complex reactions occur in steps (elementary reactions), where each step has 1 or 2 reactants with low Ea o intermediate forms in early step and consumed later o catalyst consumed in slow step and produced later step 1: 2 Br- + 2 H+ + H2O2(aq) Br2(aq) + 2 H2O step 2: Br2(aq) + H2O2(aq) 2 Br- + 2 H+ + O2(g) overall: 2 H2O2(aq) 2 H2O + O2(g) o catalyst: H+ and Br-—included in rate law o intermediate: Br2—not included in rate law o rate = k[Br-]2[H+]2[H2O2] ClOb. For the reaction, (1) write the rate law for the reaction. (2) calculate rate constant, k, and specify units. c. What is the activation energy (Ea) for this reaction if the rate doubles when the temperature increases from 20.0oC to 30.0oC? ln(k1/k2) = (Ea/R)[1/T2 – 1/T1]