Properties of Matter
advertisement

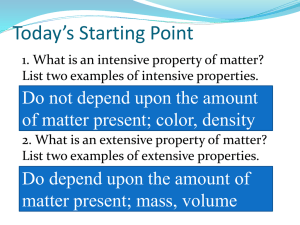
Thursday, September 13, 2013 How Can Density Be Used to Identify A substance Lab Bell Work 9/16 What is the volume of a 36 g rock if its density is 25 g/ml? What is the mass of a brick if its density is 8.9g/cm3, and the bricks dimensions are 2cm x 11cm x 8cm? Agenda Discuss Classification of Matter Complete Classification Activity Objectives Students will be able to: Classify matter into different categories Use density to identify a substance Classification of Matter Matter can be classified into many different categories. The next few slides we will be going over each of these categories Classification of Matter How are they similar? . . . Different? Only one type of matter in sample Water (H2O) Muddy Water Classification of Matter Pure Substance Only one type of matter in sample Water (H2O) Classification of Matter Pure Substances – How are they different Water (H2O) Gold (Au) Classification of Matter Pure Substances – Element Atoms of only one type are in the substance Gold (Au) Classification of Matter Pure Substances – Compound Atoms of more than one type are in the substance Water (H2O) Classification of Matter Mixture Two or more types of matter in the sample Chicken Soup Classification of Matter Mixtures – How are they different? Chicken Soup Cola Classification of Matter Heterogeneous Mixture Mixture is not the same throughout— Can identify different parts Chicken Soup Classification of Matter Homogenous Mixture Mixture is the same throughout Cola A. Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions Element no A. Matter Flowchart Examples: graphite pepper • Element • Hetero. Mixture sugar (sucrose) • Compound paint • Hetero. Mixture soda • Homo. Mixture B. Pure Substances Element composed of identical atoms EX: copper wire, aluminum foil B. Pure Substances Compound composed of 2 or more elements in a fixed ratio properties differ from those of individual elements EX: table salt (NaCl) Compound Example C. Mixtures Variable combination of 2 or more pure substances. Heterogeneous Homogeneous Tyndall Effect https://www.youtube.com/watch?v=S oUmz8L87Z4 C. Mixtures Solution homogeneous very small particles no Tyndall effect particles don’t settle EX: rubbing alcohol Tyndall Effect C. Mixtures Colloid heterogeneous medium-sized particles Tyndall effect particles don’t settle EX: milk C. Mixtures Suspension heterogeneous large particles Tyndall effect particles settle EX: fresh-squeezed lemonade C. Mixtures Examples: mayonnaise colloid muddy water suspension fog colloid saltwater solution Italian salad dressing suspension Physical vs. Chemical Properties Physical Property Can be observed without changing the identity of the substance Chemical Property Describes the ability of a substance to undergo changes in identity B. Physical vs. Chemical Properties Examples: melting point physical flammable chemical density physical magnetic physical tarnishes in air chemical Physical vs. Chemical Change Physical Change Changes the form of a substance without changing its identity Properties remain the same Reversible Chemical Change Changes the identity of a substance Products have different properties Irreversible Physical vs. Chemical Change Signs of a Chemical Change change in color or odor formation of a precipitate (solid) formation of a gas change in light or heat Physical vs. Chemical Change Examples: rusting iron chemical dissolving in water physical burning a match chemical melting ice physical grinding salt physical Classifying Matter Activity Get out a sheet of paper and put your name on the top. Create a data chart with the following headings Vial Number Name of Material Pure Substance or Mixture Element, Compound, Hetero or Homo geneous Reasoning (Why did you classify it as this?) Make 20 rows and label 1-20 in the column “vial number” Classifying Matter Activity You will pair up and sit with your partner on one side of each lab table with 1 group at the back table if needed. I will come give you a vial with matter in it, you will write the name of the matter in the appropriate column, decide whether it is a pure substance or a mixture and then what type of pure substance or mixture. Then pass it on to the next group Analysis 1. Which classification was hardest to determine- Element, Compound, Heterogeneous or Homogenous? Why was this the hardest one? 2. If you had to separate the heterogeneous mixtures into colloids or suspensions, what property would you look at to separate the two? 3. Determine whether each sample below is an element, compound, heterogeneous or homogeneous mixture A B C D E